Abstract
Spinal osteophytic changes are known to be affected by differences in age, sex, population, and mechanical stress. We examined Joseon skeletons (n=87) to obtain vertebral osteophytosis data on a pre-modern Korean population. The mean osteophytic value (MOV) of vertebrae increased in the cervical-thoracic-lumbar order. More severe osteophytosis was found in the vertebrae (C5, T9, T10, and L4) farthest from the line of gravity, while the general pattern of vertebral osteophytosis appeared similar to those of previous reports on other skeletal series. More severe osteophytes were much more common in the males, possibly due to their engaging in more strenuous physical labor than that of females. We also observed MOV patterns seemingly unique to the Joseon people, and findings not typically reported in previous studies. Although a full explanation of the factors contributing to vertebral-osteophytic development in Joseon Koreans will require further studies, the present results are meaningful to anatomists and anthropologists interested in osteophytic patterns occurring in an East Asian population.
A spinal osteophyte is defined as a fibrocartilage-capped, bony outgrowth in the vertebral body [1, 2]. It is known to develop in response to continual pressure on the vertebral body, inducing collapse of the intervertebral disc and a general weakening of the vertebral column; therefore, manifesting finally as nerve compression and limited joint mobility [1-6]. Anatomists and anthropologists have investigated correlations between degenerative changes in the spine and various environmental, biological, and genetic factors [7-17]. For example, sex was found to affect the vertebral osteophytes in a British skeletal collection [17]. Vertebral osteophytes are also known to be affected by aging. The role of mechanical stress in the formation of spinal degenerative changes is another common focus among researchers [11]. Of course, not only mechanical loading but also repetitive movement and gravitational stress caused by human bipedality are associated with the development of vertebral osteophytes [6, 11, 14, 15, 18-20].
In addition, differences in the human population are likely to be another important contributing factor because a comparison of many studies on various human societies shows different prevalences of vertebral osteophytes [11, 14-17, 21, 22]. However, information on vertebral osteophytes in different human populations is insufficient to form a conclusion. In particular, there is a relative lack of studies on vertebral ostephytes in historical skeletal series from the East Asian region (the one exception is the report on Japanese skeletons by Shimoda et al. [23]). Over the past several years, we have sought to fill this research gap by building a human skeletal series comprised of specimens discovered in pre-modern Korean tombs dating to the Joseon Dynasty (1392-1910). We undertook an anthropological examination on the vertebral osteophytes in a Joseon sample, which will be significant to researchers interested in vertebral osteophytes in different human populations.
We examined the Joseon people's vertebrae maintained at Seoul National University College of Medicine, Korea. Sex was determined by the shapes of the sciatic notch or the mastoid process. Age at death was estimated by auricular-surface degeneration of the hip bone. Age at death was categorized into eight phases based on transverse organization, granularity, apical activity, degeneration of the retroauricular area, and porosity of the auricular surface [24]: 1-2 as young (20-35 years), 3-6 as middle (36-50 years), and 7-8 as older adult (>50 years). Following previous studies [6, 22], the first cervical vertebra (C1) was excluded because it does not have a vertebral body to assess. Among 101 individuals, four cases with vertebrae showing pathological signs of diffuse idiopathic skeletal hyperostosis or ankylosing spondylitis [25] were also excluded. Ten cases showing severe taphonomic changes in anterior margins of vertebral bodies were also excluded. Thus, a total 1,578 vertebrae from 87 Joseon individuals were finally investigated. The sex of all 87 cases was determined, whereas age at death was uncertain in two.
Osteophytes were diagnosed when bony outgrowths developed on the margins of vertebral bodies [4, 7, 26]. The degree of vertebral osteophytic damage was classified into five stages (0-4) based on the methods of Snodgrass [27] and Van der Merwe [6]. Briefly, grade 0 shows no indication of osteophytosis (i.e., no lipping on the margin); grade 1 has a single osteophyte point visible on the vertebral body, with slight lipping on the superior and inferior margins; grade 2 exhibits more lipping on the margins, projecting almost horizontally from the vertebral body; grade 3 exhibits advanced lipping, assuming a bird's beak shape, with the free end curving in the direction of the closest intervertebral space; and grade 4 is assigned to osteophytes of two or more adjacent vertebrae fused together [4, 7, 26, 27] (Fig. 1). The mean osteophytic value (MOV) was defined as the mean osteophytic grade value for each vertebra to compare osteophytosis severity in each vertebral segment.
Statistical analysis was performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). Frequencies, means, and standard deviations of data were calculated and compared between the groups using the t-test or analysis of variance. The Kruskal-Wallis test [28] was used to test for differences in quantitative parameters in the small groups. Differences in the frequency of osteophyte development were tested by the chi-square test or Fisher's exact test [29]. A P<0.05 was considered significant.
Among the 87 Joseon skeletal specimens examined, 49 (56.3%) and 38 (43.7%) were determined to be male and female, respectively. The age at death could not be determined, with the exceptions of two cases (nos. 179 and 223).Thirteen (14.9%), 42 (48.3%), and 30 (34.5%) individuals were classified as young, middle, and older aged, respectively (Electronic Supplementary Table 1). Cervical, thoracic, and lumbar osteophytosis was observed in 82.1% (69/84), 94.7% (74/76), and 90.7% (68/75) of the cases (Table 1). When a stage 2 osteophyte was set as a cut-off criterion, the prevalences were adjusted to 32.1% (27/84), 67.1% (51/76), and 73.3% (55/75), respectively. The osteophytic difference by sex was not statistically significant (males, 98.0%, 48/49; females, 100%, 38/38; P>0.05).
We also assessed osteophyte severity using the MOV (Fig. 2, Electronic Supplementary Table 2). As a result, the MOV increased in the cervical-thoracic-lumbar order, except for C6-T1 and T10-T11 (Fig. 2A). Only 7.1% and 26.7% of the cervical and lumbar spines, respectively, showed grade 3 vertebral osteophytes (Electronic Supplementary Table 2). The MOV distribution pattern was similar in the cervical, thoracic, and lumbar segments, whereas the osteophytic grades rose towards the vertebrae in the middle (Fig. 2B). The highest MOV was that for C5 (mean, 1.31 for males and 0.74 for females) among the cervical vertebrae, T9 (mean, 1.35 for males) and T10 (mean, 1.00 for females) among the thoracic vertebrae, and L4 (mean, 1.85 for males and 1.44 for females) among the lumbar vertebrae. The lowest MOV was that for C2 (mean, 0.33 for males and 0.16 for females), T1 (mean, 0.46 for males and 0.60 for females), and L1 (mean, 1.41 for males and 0.71 for females) (Electronic Supplementary Table 3).
The male and female vertebrae in each segment showed similar distribution patterns (Fig. 3): the highest MOVs were those for C5, T9 (males)/T4, and T10 (females) and L4, which are the most distant vertebrae from the line of gravity. In contrast, MOV was the lowest in the cases of T1 and T2 where the spinal column was in the center of the line of gravity (Fig. 3). However, the MOV pattern of females was slightly different from that of males. Specifically, a bimodal pattern of MOV (T4 and T10) was observed in the female thoracic vertebrae. In general, the Joseon males showed a higher MOV than their female counterparts (Table 2). In a comparison of the MOV by sex, ostetophytes in C5, T7, T8, T12, L1, and L2 were statistically more prevalent among males than among females (P<0.05) (Electronic Supplementary Table 3). Additionally, severe cases were much more commonly observed in males (Electronic Supplementary Table 4). We also tested for osteophytes at each spinal level by age group. The cervical and lumbar frequencies for the middle and older age groups were significantly higher than for the young-age group (Table 3). Overall, the older the age group, the higher the MOV (Fig. 4, Electronic Supplementary Table 5).
Osteophyte distributions along the vertebral column have been well documented in previous reports. Osteophytic severity is associated with gravity; specifically, greater pressure is loaded on vertebrae farthest from the line of gravity [6]. This general pattern of vertebral osteophytes was not only observed in the present study, but has been featured in previously reported findings as well.
A number of studies have also found osteophytic development to be very commonly associated with secondary curvature of the spine (i.e., of the cervical and lumbar vertebrae) [6, 30, 31]. For example, osteophytic severity among a South African skeletal series was highest in the lumbar, followed by the cervical, and then the thoracic vertebrae [6]. The thoracic vertebrae exhibited relatively less osteophytic development despite that some of them were located farthest from the line of gravity, possibly due to the stability provided by the supportive action of adjacent ribs [6, 31]. However, such an overall osteophytic pattern remains controversial, as some skeletal series have shown discordant patterns. For example [32, 33], osteophytic severity involving the thoracic vertebrae was not significantly lower in historical Croatian and English samples than those with cervical and lumbar osteophytosis (Electronic Supplementary Table 6). Furthermore, the MOV of the thoracic vertebrae in our Joseon skeletons farthest from the line of gravity (T9, mean, 1.35 for males; T10, mean, 1.00 for females) was higher than values for their counterpart cervical vertebrae (C5, mean, 1.31 for males and 0.74 for females) but lower than those of the lumbar vertebrae (L4, mean, 1.85 for males and 1.44 for females).
In fact, vertebral degenerative changes correlate with occupational-related stressors. For example, vertebral changes observed in skeletons taken from the sunken 16th century English battleship Mary Rose were thought to have been caused by official activities undertaken by the crew members [21]. Similarly, we should consider the commonly employed A-frame (Jige in Korean), which was quite a convenient tool for Joseon society laborers during that period in history, but imposed a heavy load on thoracic vertebrae (Fig. 5). It is certainly possible that this is what caused the higher thoracic MOV findings in the present Joseon skeletal series. However, this explanation invites some skepticism, as most of the samples examined in this study are suspected by archaeologists to have been upper-class members of Joseon society [34]. That is, individuals in this study would have performed significantly less physical labor than other, lower class Joseon people. Therefore, an alternative explanation for the relatively higher thoracic MOV in our Joseon skeletal series is necessary.
The prevalence of thoracic osteophytosis increases naturally with age. Indeed, some anatomists and anthropologists argue that skeletal thoracic-osteophyte findings could be used for age-at-death estimations [7, 27]. In any case, any skeletal series that includes remains of exclusively or mostly elderly individuals should show higher prevalences of vertebral osteophytosis. Therefore, we cannot rule out the possibility that the higher-thoracic-MOV skeletal series examined in this study included a greater number of specimens from older individuals than other, perhaps more typical series reported previously. This would also explain why the osteophytic frequency in our Joseon skeletal series (98.9%) was remarkably higher than figures previously reported for English, American, or Japanese historical populations (56.6-88.2%) (Table 4). The lack of satisfactory technique for estimating age at death in adult skeletons from archaeological sites may have contributed to our findings. The probable underestimation of age at death, particularly in Asian samples and elderly individuals, due to the limitations of age estimation method by Lovejoy et al. [24], may have accentuated the difference in the osteophyte prevalence compared with previous reports [35-37].
However, regardless of the true source of the present study's higher thoracic vertebral MOV, any conclusions drawn at this time must be considered provisional, because the population structures of all relevant historical skeletal series have yet to be compared in full detail.
Significant osteophytic findings for male specimens are probably due to their typical engagement in more strenuous physical labor than females [38, 39]. Although spinal columns of the two sexes show a very similar osteophytic development pattern, there are also some differences. The vertebrae located farthest from the line of gravity have more osteophytes in females than those closest. In contrast, the osteophytes in T2 to L5 show a gradual increase in development in males, and only a slight reduction in frequency at L1 is noted [6].
In our study on the Joseon skeletal series, we observed a somewhat different pattern. First, the Joseon male vertebrae located farthest from the line of gravity exhibited the highest MOV (i.e., C5, T9, and L4); that is, there was no pattern of gradual increase from the thoracic to the lumbar vertebrae. The Joseon female's osteophytic distribution generally differed from that of the South African females. For example, T4 (mean, 0.90) showed the highest MOV in the upper part of the thoracic segment, reflecting the bimodal pattern of thoracic MOV seen for Joseon females. Moreover, the female's MOV for the lower thoracic and upper lumbar vertebrae (the T-L junction) was lower than that in their male counterparts. This phenomenon is interesting, as the vertebral curvature of female is significantly posterior to the line of gravity relative to a male, thereby inducing ostephytosis in the T-L junction much more readily [40]. As this finding differed from the established hypothesis, we should consider that less strenuous physical activity and more moderate postural loads alleviated the development of osteophytes in Joseon females.
Taken together, our vertebral osteophytic findings herein on a Joseon skeletal-sample series, one of the rare such reports from any Asian countries, show a general pattern similar to those of previous studies on other, non-Asian historical populations. More severe osteophytosis was observed in the vertebrae (C5, T9, and T10 and L4) farthest from the line of gravity. Comparing the MOV by sex at each vertebra, the males showed higher values than the females, possibly because they engaged in considerably more strenuous physical labor. We also observed some unique MOV patterns not usually seen in other studies. Although a full explanation of the factors contributing to vertebral osteophytic development in Joseon Koreans will require multidisciplinary studies, the present results will be meaningful to anatomists and anthropologists interested in osteophytic patterns occurring in a non-European population.
Figures and Tables
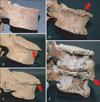 | Fig. 1Examples of osteophyte grading in this study. Lumbar vertebrae in (A-E) represent grades 0-4 respectively. (A) Grade 0: no indication of osteophytosis. (B) Grade 1: an osteophyte with slight lipping (indicated by arrow) on the margin. (C) Grade 2: more lipping (arrow) visible on the margins. (D) Grade 3: advanced lipping with the free end curving in the direction of the closest intervertebral space (indicated by arrow). (E) Grade 4: osteophytes of two or more adjacent vertebrae fused together. |
 | Fig. 2(A) Mean osteophytic value in cervical, thoracic, and lumbar vertebrae of Joseon skeletons. (B) Mean osteophytic value at a glance from the cervical to lumbar spine. |
 | Fig. 5(A) A-frame (Jige in Korean) traditionally used in premodern Korean society. (B) As a heavy burden could be carried using an A-frame, a heavy, strenuous load (indicated by arrow) occurred on the thoracic vertebrae. |
Acknowledgements
This study was supported by grant number 0420070940 from Seoul National University Hospital (SNUH) research fund. The authors would like to thank Joon Yong Sim (Goyang City Hall) for his constructive and detailed comments that help improve this manuscript.
References
1. van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007. 15:237–244.
2. Klaassen Z, Tubbs RS, Apaydin N, Hage R, Jordan R, Loukas M. Vertebral spinal osteophytes. Anat Sci Int. 2011. 86:1–9.
3. Shore LR. Polyspondylitis marginalis osteophytica. Br J Surg. 1935. 22:850–863.
4. Nathan H. Osteophytes of the vertebral column: an anatomical study of their development according to age, race, and sex with considerations as to their etiology and significance. J Bone Joint Surg Am. 1962. 44:243–268.
5. Maat GJ, Mastwijk RW, Van der Velde EA. Skeletal distribution of degenerative changes in vertebral osteophytosis, vertebral osteoarthritis and DISH. Int J Osteoarchaeol. 1995. 5:289–298.
6. Van der Merwe AE, Işcan MY, Lábbè EN. The pattern of vertebral osteophyte development in a South African population. Int J Osteoarchaeol. 2006. 16:459–464.
7. Stewart TD. The rate of development of vertebral osteoarthritis in American whites and its significance in skeletal age identification. Leech. 1958. 28:144–151.
8. Jurmain RD. The pattern of involvement of appendicular degenerative joint disease. Am J Phys Anthropol. 1980. 53:143–150.
9. Ortner DJ, Putschar WG. Smithsonian Contributions to Anthropology No. 28. Identification of pathological conditions in human skeletal remains. 1981. Washington, DC: Smithsonian Institution Press.
10. Krogman WM, İşan MY. The human skeleton in forensic medicine. 1986. Springfield: Charles C Thomas Publisher, Ltd..
11. Jurmain R. Paleoepidemiology of a central California prehistoric population from Ca-Ala-329: II. Degenerative disease. Am J Phys Anthropol. 1990. 83:83–94.
12. MacLaughlin SM, Oldale KN. Vertebral body diameters and sex prediction. Ann Hum Biol. 1992. 19:285–292.
13. Bridges PS. Vertebral arthritis and physical activities in the prehistoric southeastern United States. Am J Phys Anthropol. 1994. 93:83–93.
14. Lovell NC. Spinal arthritis and physical stress at Bronze Age Harappa. Am J Phys Anthropol. 1994. 93:149–164.
15. Knüsel CJ, Göggel S, Lucy D. Comparative degenerative joint disease of the vertebral column in the medieval monastic cemetery of the Gilbertine priory of St. Andrew, Fishergate, York, England. Am J Phys Anthropol. 1997. 103:481–495.
16. Taitz C. Osteophytosis of the cervical spine in South African blacks and whites. Clin Anat. 1999. 12:103–109.
17. Sofaer Derevenski JR. Sex differences in activity-related osseous change in the spine and the gendered division of labor at Ensay and Wharram Percy, UK. Am J Phys Anthropol. 2000. 111:333–354.
18. Rogers J, Waldron T, Dieppe P, Watt I. Arthropathies in palaeopathology: the basis of classification according to most probable cause. J Archaeol Sci. 1987. 14:179–193.
19. O'Neill TW, McCloskey EV, Kanis JA, Bhalla AK, Reeve J, Reid DM, Todd C, Woolf AD, Silman AJ. The distribution, determinants, and clinical correlates of vertebral osteophytosis: a population based survey. J Rheumatol. 1999. 26(4):842–848.
20. Weber J, Czarnetzki A, Spring A. Paleopathological features of the cervical spine in the early middle ages: natural history of degenerative diseases. Neurosurgery. 2003. 53:1418–1423.
21. Stirland AJ, Waldron T. Evidence for activity related markers in the vertebrae of the crew of the Mary Rose. J Archaeol Sci. 1997. 24:329–335.
22. Rojas-Sepúlveda C, Ardagna Y, Dutour O. Paleoepidemiology of vertebral degenerative disease in a Pre-Columbian Muisca series from Colombia. Am J Phys Anthropol. 2008. 135:416–430.
23. Shimoda Y, Nagaoka T, Moromizato K, Sunagawa M, Hanihara T, Yoneda M, Hirata K, Ono H, Amano T, Fukumine T, Ishida H. Degenerative changes of the spine in people from prehistoric Okhotsk culture and two ancient human groups from Kanto and Okinawa, Japan. Anthropol Sci. 2012. 120:1–21.
24. Lovejoy CO, Meindl RS, Pryzbeck TR, Mensforth RP. Chronological metamorphosis of the auricular surface of the ilium: a new method for the determination of adult skeletal age at death. Am J Phys Anthropol. 1985. 68:15–28.
25. Rogers J, Waldron T. DISH and the monastic way of life. Int J Osteoarchaeol. 2001. 11:357–365.
26. Steinbock RT. Paleopathological diagnosis and interpretations: bone diseases in ancient human populations. 1976. Springfield: Charles C. Thomas Publisher, Ltd..
27. Snodgrass JJ. Sex differences and aging of the vertebral column. J Forensic Sci. 2004. 49:458–463.
28. Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952. 47:583–621.
29. Fisher RA. Statistical methods for research workers. 1954. Edinburgh: Oliver and Boyd.
30. Levy LF. Porter's neck. Br Med J. 1968. 2:16–19.
31. Hollinshead WH. Functional anatomy of the limbs and back. 1969. 3rd ed. Philadelphia: W. B. Saunders.
32. Novak M, Šlaus M. Vertebral pathologies in two early modern period (16th-19th century) populations from Croatia. Am J Phys Anthropol. 2011. 145:270–281.
33. Waldron T. The prevalence of, and the relationship between some spinal diseases in a human skeletal population from London. Int J Osteoarchaeol. 1991. 1:103–110.
34. Shin MH, Yi YS, Bok GD, Lee EJ, Spigelman M, Park JB, Min SR, Shin DH. Peña PA, Martín CR, Rodríguez MA, editors. How did mummification occur in bodies buried in tombs with a lime soil mixture barrier during the Joseon Dynasty in Korea. Mummies and Science World Mummies Research. 2008. Santa Cruz de Tenerife: Academia Canaria de la Historia;105–113.
35. Buckberry JL, Chamberlain AT. Age estimation from the auricular surface of the ilium: a revised method. Am J Phys Anthropol. 2002. 119:231–239.
36. Murray KA, Murray T. A test of the auricular surface aging technique. J Forensic Sci. 1991. 36:1162–1169.
37. Schmitt A. Age-at-death assessment using the os pubis and the auricular surface of the ilium: a test on an identified Asian sample. Int J Osteoarchaeol. 2004. 14:1–6.
38. Chapman FH. Vertebral osteophytosis in prehistoric populations of central and southern Mexico. Am J Phys Anthropol. 1972. 36:31–38.
39. Prescher A. Anatomy and pathology of the aging spine. Eur J Radiol. 1998. 27:181–195.
40. Pearsall DJ, Reid JG. Line of gravity relative to upright vertebral posture. Clin Biomech. 1992. 7:80–86.
Electronic Supplementary Materials
Supplementary data including six tables can be found with this article online at http://www.acbjournal.org/src/sm/acb-45-274-s001.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download