Abstract
Apoptosis inducing factor (AIF) has been proposed to act as a putative reactive oxygen species scavenger in mitochondria. When apoptotic cell death is triggered, AIF translocates to the nucleus, where it leads to nuclear chromatin condensation and large-scale DNA fragmentation which result in caspase-independent neuronal death. We performed this study to investigate the possibility that, in addition to caspase-dependent neuronal death, AIF induced neuronal death could be a cause of neuronal death in Alzheimer's disease (AD). We have found that AIF immunoreactivity was increased in the hippocampal pyramidal neurons in the Alzheimer brains compared to those of healthy, age-matched control brains. Nuclear AIF immunoreactivity was detected in the apoptotic pyramidal CA1 neurons at the early stage of AD and CA2 at the advanced stage. Nuclear AIF positive neurons were also observed in the amygdala and cholinergic neurons of the basal forebrain (BFCN) from the early stages of AD. The results of this study imply that AIF-induced apoptosis may contribute to neuronal death within the hippocampus, amygdala, and BFCN in early of AD.
The central hypotheses on the pathogenesis of Alzheimer's disease (AD) are the Amyloid and Tau hypotheses. The amyloid hypothesis is based on an imbalance between the production and clearance of β-amyloid (Aβ) in brain [1], leading to plaque formation, consequential neuronal degeneration, and dementia. The tau hypothesis states that hyperphosphorylated tau is easy to aggregate and eventually forms neurofibrillary tangles. The intracellular tangle may interrupt microtubular function and lead to neuronal degeneration and cell death [2]. Clinically, AD is characterized by progressive impairment of cognition and emotional disturbances that are strongly correlated with synaptic degeneration and death of neurons in limbic structures such as the hippocampus and the amygdala and associated regions of the cerebral cortex [3]. In particular, the hippocampus plays a key role in memory function and is severely affected from the early stage of AD. There are reports of neuronal loss in the CA1 region of the hippocampus in early onset AD brains. The neuronal loss increases significantly with the duration or severity of the disease [4]. Although the exact cause and mechanism of neuronal loss in AD is not yet clear, extensive recent literature supports oxidative stress, mitochondrial dysfunction, and calcium dyshomeostasis as early contributors in AD pathology, occurring prior to the development of detectable plaques and tangles [5-9]. Longstanding oxidative stress has been suggested as one of the important candidates for the development of AD pathology [6]. Little is known, however, about the molecular mechanisms of oxidative stress by which it mediates neuronal cell death in AD.
Apoptosis is a cell death program that is central to cellular and tissue homeostasis, and is involved in many physiological and pathological processes [10]. Apoptosis is characterized morphologically by a series of events that include cytoplasmic shrinkage, chromatin condensation, nuclear and cellular fragmentation, and the formation of apoptotic bodies. Although caspases are the main players involved in apoptosis, there are other molecules involved in the progression of the apoptotic cascade that are relevant to AD.
Apoptosis inducing factor (AIF) has been proposed to act as a putative reactive oxygen species scavenger [11]. Upon patho logical permeabilization of the outer mitochondrial membrane, mature AIF is further processed to a ~57 kDa form by activated calpains and/or cathepsins [12]. The soluble proapoptotic form of AIF is now called truncated AIF (tAIF). This isoform is translocated to the nucleus via its C-terminal domain nuclear localization sequence, which leads to large-scale DNA fragmentation [12, 13], a hallmark of caspase-independent apoptosis. Although research on AIF-induced neuronal death has been focused on acute insults, evidence is mounting recently that AIF-induced neuronal death could be related to chronic neurodegenerative diseases.
The possibility that AIF-induced cell death may be involved in neuronal death in chronic neurodegenerative disease has been raised by some researchers in the past few years. One group examined AIF protein levels in cortical areas of the human brain at various ages and in AD [14]. They reported that there was no significant difference in AIF protein levels from the cortex and hippocampus of AD patients versus age-matched control brains. The same group recently reported cellular and subcellular distributions of AIF in the hippocampus, entorhinal, and medial temporal cortices in postmortem samples of late AD patients [15]. However, a focused analysis of the precise impact of AIF on cell death has not yet been studied. Further investigation is therefore required to determine the role, if AIF in AD pathology.
In order to investigate the possibility of early involvement of AIF in AD pathology, we examined the expression of AIF in the various limbic regions of the age-matched control brains and AD brains, which were classified according to progression of pathology as early AD (Braak stage I and II) and advanced AD brains (Braak stage III-VI).
AIF (sc-9416) antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Fluorescein avidin D (A-2001), avidin-biotin blocking solution (SP-2001), Vectashield (H-1000) and biotinylated anti-goat IgG (BA-5000) were supplied by Vector Laboratories (Burlingame, CA, USA). Hoechst 33342 (bis-Benzimide H33342 trihydrochloride, 14533) and DAB (3,3'-diaminobenzidine tetrahydrochloride, D-5905) were supplied by Sigma Aldrich (St. Louis, MO, USA).
Fourteen age-matched control brains with no clinical or neuropathologic evidence of AD were obtained within 24 postmortem hours from the cadavers of the Department of Anatomy and Neuroscience in the School of Medicine at Eulji University. Consent was given for brain donation by next-of-kin in all cases, and a postmortem examination was conducted by the Anatomy laboratory according to standardized protocols. All experimental procedures were performed in accordance with 'The Guidelines of the IRB (Ethics Committee) at Eulji University.' A total of fourteen brain hemispheres obtained by autopsy were fixed in 10% neutral buffered formalin. The fixed hemispheres were cut on the coronal plane, and blocks were selected from 10 to 15 brain areas for pathologic diagnosis and comprehensive evaluation of the neurodegenerative process. They were prepared according to routine histological procedures for paraffin section. The twenty-six Alzheimer brains were generously gifted from Dr. Roger A. Brumback and were diagnosed by him. All brains were processed according to same protocols. The regions were cut on the coronal plane at 8 m thicknesses for immunohistochemical study, or 20 µm for confocal microscopic immunofluorescence study, then mounted on poly-L-lysine-coated slides. The sections were deparaffinized and hydrated according to routine graded xylene-alcohol methods. Hematoxylin and eosin (H&E) and Luxol fast blue/cresyl violet (LFB-CV) stains were done for general histopathology. Modified silver stain by Campbell et al. [16] for detecting the senile plaque and Gallyas stain [17] for detecting neurofibrillary tangles (NFT) were employed. The modified Campbell's silver staining method is a combination of Campbell and Gallyas staining. The technique was carried out as previously described [18]. The detailed data for each brain is summarized in Table 1.
In order to retrieve antigenecity, dewaxed sections were boiled within 0.1 mol/L citrate-buffered saline (pH 6.0) for 10 minutes. After cooling down for 30 minutes, the sections were rinsed in phosphate buffered saline (PBS). The endogenous peroxidase was quenched by 1% hydrogen peroxide in 10% methanol for 30 minutes. After two changes of PBS-T (0.1% Triton X-100 in 0.1 mol/L PBS, pH 7.6) washing for 5 minutes each, the sections were blocked for 1 hour in blocking solution (5% rabbit serum+1% bovine serum albumin in PBS-T) and incubated in primary antibody (anti-AIF, 1 : 200) at 4℃ overnight. After PBS-T rinses, the sections were incubated in a biotinylated secondary antibody for 1 hour at room temperature (RT). After PBS rinsing and an avidin-biotin-peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories) treatment for 1 hour at RT, the sections were developed for 5 minutes in a 0.05% DAB solution. As a negative control for nonspecific staining, the sections were incubated with initial incubation media minus the primary antibody, and otherwise processed as described. Images were captured with an Axiocam digital camera (Carl Zeiss Co., Jena, Germany) attached to an Olympus AX70 microscope (Olympus, Tokyo, Japan).
To detect whether the AIF expressed in the apoptotic pyramidal neurons was translocated to the nucleus, we performed confocal immunofluorescence study on the same section. Pre-staining processes were the same as described. The sections were incubated in primary antibody (anti-AIF, 1 : 50) overnight at 4℃. They were washed and incubated with the appropriate secondary biotinylated antibody. After PBS rinsing, the sections were incubated with FITC avidin D. Then, nuclear stain was done with 10 µM Hoechst 33342 for 30 minutes. To reduce background, the sections were incubated in 10% Sudan black B solution for 5 minutes, and then rinsed with distilled, deionized water and mounted with Vectashield. The AIF immunoreactivity was visualized with FITC fluorescence (green), and nuclear stain was detected with Hoechst 33342 fluorescence (blue) under UV on the same section. The images were captured with an LSM 510 meta system (Zeiss LSM 510 laser scanning microscope, Carl Zeiss Co.).
Except for the confocal microscopic images, all images were captured using an Olympus AX70 microscope outfitted with an Axiocam digital camera. The final magnifications were 100×, 200×, and 400×, and the capture frame size was 0.3 mm2. The selected anatomical regions included hippocampus, amygdala and cholinergic neurons of the basal forebrain (BFCN). Within each region, two images were captured at evenly spaced intervals to represent the entire area of interest. The numbers of AIF-positive cells (nuclear AIF staining) were expressed as the mean number of cells per mm2 of the cross sectional area in the age-matched control, the early AD, and the advanced AD brains. To prevent a potential bias toward lower counts of neurons positive for AIF in severe AD cases resulted from neuronal loss, all counts were standardized with respect to the total number of neurons counted. Statistical analysis was conducted with Graph Pad Prism (GraphPad software, San Diego, CA, USA). For multiple group comparisons, statistical differences were calculated by oneway ANOVA followed by Dunnett's test. Values of P<0.05 were considered significant.
All brains (age-matched control and AD) were assigned according to the neuropathological staging by Braak and Braak [19]. The three groups are referred to as normal (age-matched control), and mildly (early AD) or severely affected (advanced AD), referring to the extent of NFT and senile plaque in the hippocampus. Postmortem examination confirmed the diagnosis of AD given by the presence of abundant amyloid deposits and neurofibrillary tangles, and H&E and LFB-CV staining also demonstrated marked cell loss (Fig. 1A-C).
The nuclear translocation of AIF can induce caspase-independent neuronal death [13]. We thus determined the proportion of AIF positive pyramidal neurons (displaying a nuclear AIF) versus total pyramidal neurons. No immunoreactive product was detected on the negative reaction control section (Fig. 2A, top panel). In age-matched control, AIF immunoreactivity was detected in the cytoplasm of the pyramidal neuron of the CA1-CA3 sectors (Fig. 2A). We perceived that AIF immunoreactivity in the AD brain was distinguishable from the age-matched control as AD progresses. In the early and advanced AD brains, AIF immunoreactivity was detected in the nucleus of the hippocampal pyramidal neuron in all CA sectors (Fig. 2A). We found that AIF immunoreactivity was increased in the CA1, 2, 3 sectors in AD brains as compared to age-matched control. (Fig. 2B-D). In particular, nuclear AIF immunoreactivity was notably higher in the apoptotic pyramidal neuron of the CA1 in the early AD brains and of the CA2 in the advanced AD brains (Fig. 2B, C). These results suggest that AIF-induced apoptosis is involved in AD progression.
No immunoreaction product was visible on negative control sections (data not shown). Fig. 3A and B show that in age-matched control brains, AIF was localized in cytoplasm but not in the nucleus. In early AD, AIF immunoreactivity was increased in the cytoplasm of most of the pyramidal neurons. In advanced AD brains, nuclear AIF immunoreactivity was mainly observed in cells showing nuclear condensation and pyknosis. To confirm the nuclear translocation of AIF, we performed immunofluorescence for AIF and a nuclear marker, Hoechst 33342. In early AD, AIF immunofluorescence in cytoplasm was more intense compared to the age-matched control (Fig. 3B). Nuclear AIF immunofluorescence was mainly observed in the apoptotic cells with condensed nucleuses (Fig. 3B, boxed area of the lower panel). It is supposed that the nuclear translocation of AIF could be associated with nuclear condensation and cellular shrinkage of the hippocampal pyramidal neuron in the AD brain.
AD is strongly correlated with cell loss and death of neurons in the limbic structures, such as the hippocampus and the amygdala, and the associated regions of cerebral cortex [3]. Campbell's staining confirmed amyloid deposits and NFT (for AD diagnosis). H&E and LFB-CV staining also demonstrated marked cell loss (Fig. 4A).
To further determine whether AIF immunoreactivity was changed in the corticomedial nucleus of amygdala of AD brain, we investigated that expression of AIF in those regions. The number of neurons in the corticomedial nucleus of theamygdala was decreased with AD progression (Fig. 4B). In early AD, nuclear AIF immunoreactivity was significantly increased in comparison to age-matched control (Fig. 4C). In advanced AD, nuclear AIF immunoreactivity was increased as well, but had no statistical significance.
The BFCN undergo selective loss in neurodegenerative disorders of the elderly, specifically, AD [20]. So we investigated the expression of AIF in the BFCN of AD brains. The number of basal forebrain cholinergic neurons was reduced significantly with AD progression (Fig. 5B). In age-matched control, AIF immunoreactivity was detected in the cytoplasm of large neurons which were assumed to be cholinergic (Fig. 5A). In early AD, nuclear AIF immunoreactivity significantly increased compared to age-matched control (Fig. 5C). In advanced AD, nuclear AIF immunoreactivity was increased as well, but had no statistical significance.
AD is the most common cause of dementia. Among the many other pathological hallmarks, neuronal loss is considered as one of the main causative events of dementia in this catastrophic disease. The neuronal death in AD may result directly and/or indirectly from the triggering insults caused by Aβ toxicity, glutamate excitotoxicity, long-lasting oxidative stress, DNA damage, and elevation of intracellular calcium levels [3]. Thus, the mode of cell death in AD remains a matter of controversy [21], and it is possible that both apoptotic and non-apoptotic cell death coexist in the brains of affected patients.
Apoptosis, a type programmed cell death (PCD), is not always harmful. It is widely involved in normal regulatory life events and to some extent participates in pathologic events as well. Since the duration of the apoptotic process is relatively short, only a few cells that are undergoing apoptosis can be detected at a single moment in the course of a chronic neurodegenerative disease [22]. Previously, it was generally considered that apoptotic neuronal death in chronic neurodegenerative disease, e.g., AD, Parkinson's disease, etc., is associated with classical caspase mediated cell death [23-26]. However, in part, it was suggested that the caspase-independent pathway might also participate in the pathogenesis of the disease [27, 28]. According to Yang et al. [29], occasional neurons in brain sections of older PS/APP mice displayed abnormal morphological changes such as cell shrinkage, condensed nucleus and cytoplasmic organelles, and evidence of plasmalemmal blebbing. These features and the appearance of corkscrew-like dendrites in some neurons correspond to so-called "dark neurons." On sections processed with anti-activated caspase-3 antibodies, few, if any, silver-gold particles were detected in dark neurons, indicating that caspase 3 is not activated in the dark neurons. Thus, the existence of at least two modes of neurodegeneration in the same mouse model underscores the complexity of cell death patterns in chronic neurodegenerative disease. Cross talk is extensive between different cell death pathways, which include multiple types of caspase-dependent and caspase-independent programmed cell death. AIF appears to play an important role in acute neural tissue damage induced by trauma, hypoglycemia, transient ischemia, and chronic neurodegenerative diseases. Bahi et al. [30] demonstrated that experimental ischemia in embryonic cardiomyocytes triggers caspase activation, but the mode of cell death switches into caspase-independent PCD in differentiated cardiomyocytes. Although it is the result of in vitro procedures, it indicates that the cell death mechanisms are different depending on the cell's status. Unlike in vivo, in vitro study indicates that the forms of cell death are the same and that the translocation of AIF is not caused by nuclear shrinkage [31], because AIF is clearly seen in the nucleus at early points in time when the nucleus is clearly distinguishable from cytoplasmic structures. For many years, there has been some debate about whether there is apoptotic cell death in AD and whether it is in anyway related to cognitive impairment and dementia. While the study of apoptosis and apoptotic mechanisms is fairly easy to perform in cell culture systems and animal models, it is much more difficult in human organs including the brain. A few years ago, it was demonstrated that Aβ-induced neuronal apoptosis lead to AIF translocation from mitochondria to the nucleus in embryonic rat cortical cultures [32]. Interestingly, both mitochondrial and nuclear effects of AIF have been observed in neuronal death associated with rodent aging and acute traumatic injuries [11, 33]. What was shown is consistent with a possible involvement of AIF in neuronal cell death on AD pathology. Interestingly, a study on AIF protein levels in the cortical areas of human brains at various ages and in AD was reported [14]. Recently, a study by the same group provided the first demonstrations of increased nuclear translocation of AIF and its colocalization with NFT in the AD brain in midto late stages, but not in early stages [15]. Studies on defining the plausible mechanism and role of AIF on cell death in AD human brains have not been widely performed thus far. In this article, the focus has been centered on the possibility of early involvement of AIF in neurodegeneration and cell death in AD. This study proposes that the dysfunction of neurons precedes the obvious pathological abnormalities and that the intraneuronal molecular changes caused by this stress-induced injury are subtle. The present study was noteworthy in documenting AIF protein expression in the hippocampal pyramidal neurons in human AD brains. Our study confirmed caspase 3 immunoreactivity in AD (data not shown). Our in vivo results suggest that the AIF-mediated caspase-independent apoptotic pathway associated with dark neurons may be involved in the hippocampal pyramidal neuron death from early stages of AD.
The basal forebrain contains a population of large cholinergic neurons and is subdivided into four groups, Ch1-Ch4 [34, 35]. Ch4, which is very prevalent in human brains, most closely corresponds to the nucleus basalis of Meynert which is embedded in the substantia innominata, and at least 90% of the neurons in the nucleus basalis are cholinergic. It is well known in AD that the cholinergic neurons in the nucleus basalis of Meynert undergo a profound and selective degeneration from the early stages of the disease [36, 37].
The amygdala is a gray mass situated in the dorsomedial portion of the temporal lobe. It has been known that the amygdala is involved from in early AD. The amygdala nuclear complex is divided into two main nuclear masses: a corticomedial nuclear group and a basolateral nuclear group. The cholinergic innervation of the amygdala in human is severely affected in case of AD [38]. It is still debated whether it is the reduction in cholinergic innervations or other mechanisms that directly influence on the neuronal death in the amygdala. Early studies reported that the neurons in the corticomedial nuclear group are extensively degenerated from the early stage, but the neurons in the basolateral nuclear group are not [39, 40]. In summary, in order to identify the involvement of AIF-mediated caspase-independent cell death in the pathology of AD, we investigated the AIF protein expression in the hippocampus, amygdala, and BFCN in accordance with AD progression. The major findings are that 1) AIF immunoreactivity is increased in the nucleus of hippocampal pyramidal neurons in the AD brain, and the expression of this molecule is altered in accordance with the progression of AD. 2) From the early stage of AD, AIF is translocated into the nucleus of apoptotic pyramidal neurons. 3) AIF immunoreactivity was increased in the neurons of corticomedial nucleus of the amygdala and BFCN in early AD and the neurons showing nuclear translocation of AIF were also increased in early AD in comparison to the age-matched control in those areas. Unfortunately, we do not have proper specimens for biochemical study which would be helpful in identifying the precise molecular mechanism involved in AIF induced cell death in AD, and thus our study is inevitably limited only to morphological study. Although our information is not enough to solve the exact molecular events involved, this study gives the first morphological evidence that from early stages of AD, caspase-independent neuronal death is also involved in the pathogenesis of AD. Our results may provide a novel concept for developing new therapeutic strategies against chronic neurodegenerative diseases. To identify, however, the precise molecular mechanism involved in AIF-mediated neuronal death in AD, further investigations will be required.
Figures and Tables
Fig. 1
Pathological changes of pyramidal neurons and plaques in Alzhimer's disease (AD) brains. (A) Light micrographs of the CA1 region of the hippocampus. Hematoxylin and eosin (H&E), Luxol fast blue/cresyl violet (LFB-CV) and modified Campbell's silver stain were sequentially applied to the semi-serial sections. Scale bar=100 µm. (B) Quantitative analysis of data in (A) shows neuronal loss of the hippocampal pyramidal neurons. (C) Quantitative analysis of data in (A) shows the number of plaques in CA1. n=14 for age-matched control, n=15 for early AD and n=11 for advanced AD. *P<0.05, †P<0.01.
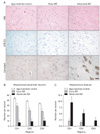
Fig. 2
(A) Apoptosis inducing factor (AIF) immunoreactivity in the CA1, CA2, and CA3 regions of the age-matched control, early Alzhimer's disease (AD), and advanced AD brains. Top panels are negative control of CA1. Scale bar=50 µm. (B-D) Quantitative analysis of incidence (% of nuclear AIF positive neurons/total neurons per section) of nuclear AIF-positive neurons in the CA1 (B), CA2 (C), and CA3 regions (D). n=14 for age-matched control, n=15 for early AD, and n=11 for advanced AD. †P<0.01.
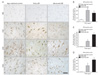
Fig. 3
Nuclear translocation of apoptosis inducing factor (AIF) in the apoptotic pyramidal neurons. (A) In age-matched control, AIF immunoreactivity is detected in the cytoplasm of pyramidal neurons. In early Alzhimer's disease (AD), the intensity of AIF immunoreactivity (boxed area) increases in cytoplasm and the nucleus as well, but nuclear morphology is not conspicuously altered. In advanced AD, the apoptotic pyramidal neurons showing nuclear AIF immunoreactivity (boxed area) are shrunken, and their nuclei are serrated, which implies nuclear damage. (B) Confocal immunofluorescence micrographs showing the nuclear translocation of AIF in the hippocampal pyramidal neurons of early AD. In age-matched control, AIF (FITC) is detected exclusively in the cytoplasm of the neurons (upper panel; boxed area). In early AD, the overlay of AIF and Hoechst 33342 (lower panel; boxed area) indicates that AIF has translocated into the nucleus. The nuclear translocation of AIF is associated with nuclear pyknosis and cellular shrinkage. Nuclei are stained with Hoechst 33342. Scale bars=100 µm (A, B), 5 µm (inset, enlarged areas of boxed area in A), 10 µm (inset, enlarged areas of boxed area in B).
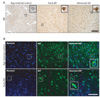
Fig. 4
Apoptosis inducing factor (AIF) immunohistochemistry in the corticomedial nucleus of the amygdala of Alzhimer's disease (AD) brains. (A) Hematoxylin and eosin (H&E), Luxol fast blue/cresyl violet (LFB-CV) and modified Campbell's silver stain and AIF immunohistochemistry of the corticomedial nucleus of amygdala. Scale bars=100 µm. (B) Quantitative analysis of the corticomedial neuron loss in (A). (C) Quantitative analysis of the incidence of the AIF-positive neurons in the corticomedial nucleus. n=14 for age-matched control, n=15 for early AD, and n=11 for advanced AD. *P<0.05, †P<0.01.
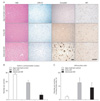
Fig. 5
Apoptosis inducing factor (AIF) immunohistochemistry in the cholinergic neurons of the basal forebrain (BFCN) of Alzhimer's disease (AD) brains. (A) Hematoxylin and eosin (H&E), Luxol fast blue/cresyl violet (LFB-CV) and modified Campbell's silver stain and AIF immunohistochemistry of the BFCN. Scale bars=100 µm. (B) Quantitative analysis of the neuronal loss in BFCN in (A). (C) Quantitative analysis of the incidence of the AIF positive neurons in the BFCN. n=14 for age-matched control, n=15 for early AD, and n=11 for advanced AD. *P<0.05, †P<0.01.
Acknowledgements
We thank Dr. Roger A. Brumback (Department of pathology, School of Medicine, Creighton University, Omaha, Nebraska, USA) for providing AD human brain blocks.
This work was supported by the Converging Research Center Program through the National Research Foundation of Korea (NRF) (2009-0082268).
References
1. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002. 297:353–356.
2. Mudher A, Lovestone S. Alzheimer's disease: do tauists and baptists finally shake hands? Trends Neurosci. 2002. 25:22–26.
3. Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004. 430:631–639.
4. Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001. 58:1395–1402.
5. Nunomura A, Chiba S. Avoidance of apoptosis in Alzheimer's Disease. J Alzheimers Dis. 2000. 2:59–60.
6. Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001. 60:759–767.
7. Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J Neurosci. 1999. 19:1959–1964.
8. Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001. 21:4183–4187.
9. Lee HG, Castellani RJ, Zhu X, Perry G, Smith MA. Amyloid-beta in Alzheimer's disease: the horse or the cart? Pathogenic or protective? Int J Exp Pathol. 2005. 86:133–138.
10. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001. 411:342–348.
11. Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002. 419:367–374.
12. Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005. 24:1375–1386.
13. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.
14. Reix S, Mechawar N, Susin SA, Quirion R, Krantic S. Expression of cortical and hippocampal apoptosis-inducing factor (AIF) in aging and Alzheimer's disease. Neurobiol Aging. 2007. 28:351–356.
15. Yu W, Mechawar N, Krantic S, Quirion R. Evidence for the involvement of apoptosis-inducing factor-mediated caspase-independent neuronal death in Alzheimer disease. Am J Pathol. 2010. 176:2209–2218.
16. Campbell SK, Switzer RC, Martin TL. Alzheimer's plaques and tangles: a controlled and enhanced silver staining method. Soc Neurosci Abstr. 1987. 13:678.
17. Gallyas F. Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung. 1971. 19:1–8.
18. Woo RS, Lee JH, Yu HN, Song DY, Baik TK. Expression of ErbB4 in the neurons of Alzheimer's disease brain and APP/PS1 mice, a model of Alzheimer's disease. Anat Cell Biol. 2011. 44:116–127.
19. Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991. 1:213–216.
20. Geula C, Mesulam MM. Terry RD, Katzman R, Blick KL, Sisodia SS, editors. Cholinergic systems in Alzheimer's disease. Alzheimer Disease. 1999. 2nd ed. New York: Raven Press;269–292.
21. Jellinger KA. Cell death mechanisms in neurodegeneration. J Cell Mol Med. 2001. 5:1–17.
22. Cotman CW, Su JH. Mechanisms of neuronal death in Alzheimer's disease. Brain Pathol. 1996. 6:493–506.
23. Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004. 36:381–386.
24. Galvan V, Chen S, Lu D, Logvinova A, Goldsmith P, Koo EH, Bredesen DE. Caspase cleavage of members of the amyloid precursor family of proteins. J Neurochem. 2002. 82:283–294.
25. Takuma H, Tomiyama T, Kuida K, Mori H. Amyloid beta peptide-induced cerebral neuronal loss is mediated by caspase-3 in vivo. J Neuropathol Exp Neurol. 2004. 63:255–261.
26. Zhao M, Su J, Head E, Cotman CW. Accumulation of caspase cleaved amyloid precursor protein represents an early neurodegenerative event in aging and in Alzheimer's disease. Neurobiol Dis. 2003. 14:391–403.
27. Bertrand E, Brouillet E, Caillé I, Bouillot C, Cole GM, Prochiantz A, Allinquant B. A short cytoplasmic domain of the amyloid precursor protein induces apoptosis in vitro and in vivo. Mol Cell Neurosci. 2001. 18:503–511.
28. Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K, Lassmann H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease: evidence for apoptotic cell death. Am J Pathol. 1999. 155:1459–1466.
29. Yang DS, Kumar A, Stavrides P, Peterson J, Peterhoff CM, Pawlik M, Levy E, Cataldo AM, Nixon RA. Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer's disease. Am J Pathol. 2008. 173:665–681.
30. Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem. 2006. 281:22943–22952.
31. Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci. 2004. 24:10963–10973.
32. Movsesyan VA, Stoica BA, Faden AI. MGLuR5 activation reduces beta-amyloid-induced cell death in primary neuronal cultures and attenuates translocation of cytochrome c and apoptosis-inducing factor. J Neurochem. 2004. 89:1528–1536.
33. Zhang X, Chen J, Graham SH, Du L, Kochanek PM, Draviam R, Guo F, Nathaniel PD, Szabó C, Watkins SC, Clark RS. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J Neurochem. 2002. 82:181–191.
34. Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983. 10:1185–1201.
35. Perry RH, Candy JM, Perry EK, Thompson J, Oakley AE. The substantia innominata and adjacent regions in the human brain: histochemical and biochemical observations. J Anat. 1984. 138(Pt 4):713–732.
36. Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982. 215:1237–1239.
37. Nagai T, McGeer PL, Peng JH, McGeer EG, Dolman CE. Choline acetyltransferase immunohistochemistry in brains of Alzheimer's disease patients and controls. Neurosci Lett. 1983. 36:195–199.
38. Emre M, Heckers S, Mash DC, Geula C, Mesulam MM. Cholinergic innervation of the amygdaloid complex in the human brain and its alterations in old age and Alzheimer's disease. J Comp Neurol. 1993. 336:117–134.
39. Grunthal E. Pathological analysis of the symptoms of Alzheimer's disease. Pscychiatr Neurol Wochenschr. 1928. 36:401–407.
40. Hopper MW, Vogel FS. The limbic system in Alzheimer's disease: a neuropathologic investigation. Am J Pathol. 1976. 85:1–20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download