Abstract
In the developing human musculoskeletal system, cell death with macrophage accumulation occurs in the thigh muscle and interdigital area. To comprehensively clarify the distribution of macrophages, we immunohistochemically examined 16 pairs of upper and lower extremities without the hip joint (left and right sides) obtained from 8 human fetuses at approximately 10-15 weeks of gestation. Rather than in muscles, CD68-positive macrophages were densely distributed in loose connective tissues of the flexor aspects of the extremities, especially in the wrist, hand and foot. In contrast, no or fewer macrophages were evident in the shoulder and the extensor aspects of the extremities. The macrophages were not concentrated at the enthesis of the tendon and ligament, but tended to be arranged along other connective tissue fibers. Deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling revealed apoptosis in the hand lumbricalis muscles, but not in the area of macrophage accumulation. Likewise, podoplanin-positive lymphatic vessels were not localized to areas of macrophage accumulation. Re-organization of the connective tissue along and around the flexor tendons of the hand and foot, such as development of the bursa or tendon sheath at 10-15 weeks, might require the phagocytotic function of macrophages, although details of the mechanism remain unknown.
Macrophages play a central role in the processes of inflammation, tissue remodeling and repair in adult tissues. In inflammation, the tendon-bone interface and the synovial membrane show a common immunological response as an enthesis organ [1]. Moreover, in fetuses as well as adults, lymphangiogenesis is followed by macrophage accumulation, as macrophages themselves can transdifferentiate into lymphatic endothelium (reviewed by Kerjaschki [2]). However, in fetuses, this surprising phenomenon seemingly occurs only in loose tissue in the human fetal knee [3].
In the musculoskeletal system of embryos and fetuses, cell death with macrophage accumulation has been well described not only in the interdigital area of the foot and hand [4-6] but also in developing muscles, including those of humans (rat diaphragm [7], human quadriceps femoris muscle [8], chick neck muscles [9], murine back muscles [10] and multiple human muscles [11]). However, fetal muscle cell death is not an obligate component of morphogenesis, but a phenomenon caused by neuronal cell death and/or elimination of some muscle fiber types (reviewed by McClearn et al. [9]). Consequently, the aim of the present study was to examine comprehensively the distribution of CD68-positive macrophages in developing human extremities. We also examined the hypothetical relationship between macrophages and lymphangiogenesis. Fetal lymphatic vessels were identified with immunohistochemistry of D2-40 or podoplanin (see Materials and Methods).
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined 32 extremities obtained from 8 human mid-term fetuses at approximately 11-15 weeks of gestation: 2 fetuses at 10 weeks (crown-rump length [CRL], 50 and 55 mm), 2 fetuses at 12 weeks (CRL, 90 and 95 mm), and 4 fetuses at 15 weeks (CRL, 100, 115, 125, and 130 mm). With the agreement of the families concerned, these specimens were donated to the Department of Anatomy, Chonbuk National University, Korea, and their use for research approved by the university ethics committee. Without contravening the regulations of the respective universities or hospitals, authors other than those affiliated to Chonbuk University were waived of the need to obtain permission for this research project from the corresponding committee in Japan. All fetuses had been obtained by induced abortions. After abortion, each of the mothers had been personally informed by an obstetrician about the possibility of donating the fetus for research: no attempt was made to encourage donation. Because of randomization of the specimen numbering, it was not possible to trace any of the families concerned.
The donated fetuses were fixed with 10% v/v formalin solution for more than 3 months. After division into the head and neck, thorax, abdomen and pelvis, and the four extremities, all parts were decalcified by incubating them at 4℃ in 0.5 mol/l ethylenediaminetetraacetic acid solution (pH 7.5, Decalcifying Solution B, Wako, Tokyo, Japan) for 1-3 days, depending on the size of the material. The scapula and its associated muscles were included in the upper extremity segment, whereas the hip joint was included in the abdomen and pelvis segment for the other studies. Routine procedures for paraffin-embedded histology (using sections 5 µm thick) were conducted: the left or right extremities were used for transverse sections, while the other sides were used for longitudinal sections. Parts other than the extremities were used for our recent studies, most details of which have already been published. Most of the sections were stained with hematoxylin and eosin (H&E), but some were used for immunohistochemistry as well as terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL).
The primary antibodies used were 1) rabbit monoclonal anti-human CD68 (1 : 100, Dako, Glostrup, Denmark) and 2) mouse monoclonal anti-human D2-40 (1 : 100, Nichirei, Tokyo, Japan). The monoclonal antibody D2-40 raised against a MW 40 KD membrane sialomucin and the molecule is identical to podoplanin that expresses specifically in the lymphatic endothelium [12]. The D2-40 antibody was used after immersion in a ligand activator (Histofine SAB-PO kit, Nichirei, Tokyo, Japan) with autoclave treatment (105℃, 10 minutes). The second antibody (Dako Chem Mate Envison Kit, Dako) was labeled with horseradish peroxidase (HRP) and antigen-antibody reactions were detected using the HRP-catalyzed reaction with diaminobenzidine (with hematoxylin counterstaining). Podoplanin immunohistochemistry was conducted on 4 larger specimens (2 specimens at 12 weeks, 2 at 15 weeks). In addition, to identify cell death with DNA fragmentation in sections of the hand and wrist, we conducted TUNEL staining using a Millipore ApopTag Plus Peroxidase in situ Apoptosis Kit (Merck, Darmstadt, Germany).
The density of CD68-positive macrophages showed a clear site-specific difference: in all specimens for all developmental stages examined, CD68-positive macrophages were concentrated in the flexor aspects of the wrist, hand and foot (Figs. 1, 2, 3, 4). In the foot, the positive cells were scattered near and along the tendons of the flexor digitorum longus and flexor hallucis longus (Fig. 1B), in the plantar intermuscular connective tissues (Fig. 2B, F), and in the sinus tarsi. The thick tendon of the peroneus longus did not appear to be associated with larger numbers of CD68-positive macrophages than those accompanying the aforementioned two flexors. In the wrist and hand, abundant positive cells were arranged along the fibrous sheath of the carpal tunnel (Figs. 2D, 3C, 4B). Some were scattered around the flexor tendons within the tunnel, as well as the narrow spaces between the carpal bone and along the interosseus muscles. However, in the extensor aspects of the extremity, CD68-positive macrophages accumulation was small and limited to the connective tissue between the carpal bones (Fig. 3B) and along the abductor pollicis brevis muscle of the wrist (Fig. 4C). Other than the sheath of the primitive carpal tunnel, no distinct ligaments carried CD68-positive macrophages in the wrist, hand and foot. The joint cavity and its lining synovium of the hand and foot did not contain CD68-positive macrophages.
Proximal sites in the extremity contained fewer CD68-positive macrophages than the hand and foot. At the elbow, we found abundant CD68-positive macrophages in the annular ligament of the radius in 2 of 4 specimens at 15 weeks (Fig. 4E), but the medial collateral ligament did not show this feature. In the shoulder, small numbers of CD68-positive macrophages were evident along the tendons of the biceps brachii, subscapularis and/or infraspinatus muscles (figures not shown). In the knee, two of four specimens at 15 weeks carried CD68-positive macrophages in the loose connective tissue along the tendons of the hamstrings and the gastrocnemius medial head, although osteoclasts were also positive in the femur and tibia at this stage (Fig. 5B-D). CD68-positive macrophages were much more numerous in the elbow than in the knee and shoulder. In the calf, CD68-positive macrophages were concentrated in the medial intermuscular connective tissue of only one specimen (10 weeks) (Fig. 5F). None of the CD68-positive macrophages were located along arteries, veins and nerves (Figs. 2D, F, 4F and 5F), but appeared to be attached to connective tissue fibers. The joint cavity and its lining in the elbow, shoulder and knee were unaccompanied by CD68-positive macrophages (Figs. 4C, E, 5D).
Intramuscular CD68-positive macrophages were not common, but were evident in specific muscles: the distal end of the brachialis muscle at the elbow in 4 specimens (2 specimens at 10 weeks, 1 at 12 weeks and 1 at 15 weeks) (Fig. 1D), the pronator quadrate muscle in 2 specimens (1 specimen at 10 weeks and 1 at 15 weeks) (Fig. 4D), the supinator muscle (1 specimen at 10 weeks, 3 of 4 specimens at 15 weeks) (Fig. 4F), and the deltoideus muscle (1 specimen at 15 weeks) (figure not shown). Among these muscles, the brachialis muscle showed the highest number and density of CD68-positive macrophages. Intramuscular CD68-positive macrophages tended not to be concentrated either along the nerves, vessels or myotendinous junctions, but were distributed near the muscle margins or surfaces. Pathological hemorrhage, possibly resulting from the abortion procedure, was often evident in the muscles and subcutaneous layer (e.g., the subcutaneous layer shown in Figs. 2E and 5A), but few CD68-positive cells were present in the areas of bleeding. We did not examine the hip joint or its surrounding structures.
Although TUNEL staining was performed using sections of the hand and wrist of all specimens, the positive reaction was limited to tendons of the flexor digitorum profundus muscle and their associated lumbricalis muscle in the carpal tunnel (Fig. 2G). Podoplanin immunoreactivity was seen in 2 of 4 specimens examined. Most of the positive lymphatic vessels were located in the subcutaneous loose connective tissue, and did not overlap with the areas of CD68-positive macrophage accumulation (Fig. 3A). Most of the cartilages and some of the connective fibers, such as the thick sheath of the carpal tunnel, were also positive for podoplanin.
The present study revealed a site-dependent difference in the density of CD68-positive macrophage distribution in human fetal extremities. The stage examined was much later than the stage at which interdigital apoptosis occurs in the hand and foot [4-6]. In fetuses, accumulation of CD68-positive macrophages in specific muscles has been reported [7-10] (for more details, see Introduction). In the present study also, intramuscular CD68-positive macrophages were evident in limited sites such as the distal end of the brachialis muscle. Masson trichrome staining demonstrates abundant, dark red-colored, thick muscle fibers, especially in the brachialis muscle, at 12-15 weeks of gestation (unpublished data): these muscle fibers may be in a state of degeneration. At this stage, the nerve supply to muscles has already been established. Thus, the process of muscle degeneration does not seem to target specific muscle fiber types according to nerve supply, but rather appears to represent "adjustment" or "correction" of the muscle mass (i.e., volume and shape) according to the topographical anatomy, e.g., a decrease of muscle thickness to form the terminal part of the brachialis muscle in the narrow cubital fossa. If so, this manner of development, i.e., "build and scrap," is similar to the well known mechanism responsible for forming the interdigital space at the early stage. Using human fetuses, Abe et al. [13] demonstrated that maturation of intermediate filaments in muscle fibers starts at the myotendinous junction and expands to the mid-portion of a muscle. Although the stages of muscle maturation examined in that study partly overlapped that of the present materials (10 weeks), the intramuscular CD68-positive macrophage distribution did not suggest any relationship between intermediate filament maturation and macrophages.
However, rather than in the muscle itself, the CD68-positive macrophage accumulation was more evident in loose connective tissues, especially those in the flexor aspects of the wrist, hand and foot. In these areas at these stages, multiple tendons increase in thickness, the sheath or bursa begins to develop, and re-organization of the topographical relationship between tendons may occur. Joint cavitation has already been completed. In contrast, new connective tissue spaces, such as the carpal tunnel and the sinus tarsi, appear. The new spaces in the flexor aspects are much larger than those in the extensor aspect. Macrophages seem to play a critical role in the re-organization of soft tissues at flexor sites, although details of the mechanism involved remain unknown. Sbernardori et al. [14] reported a role of mechanical stress in histogenesis of the flexor tendon pulley system in the human embryonic hand. However, they did not discuss the possible role of cell death or macrophages. The suggested mechanism of adaptation of connective tissue seems to be quite different from that observed in adult tendons or experimental models [15, 16]. Any difference in CD68-positive macrophage number between distal and proximal sites in the extremity may depend on the stage at which examination is performed: the shoulder may have higher numbers in the earlier stage than was the case in the present materials. Likewise, CD68-positive macrophages seem to play a role in maturation of the tendon-bone interface or enthesis, possibly at stages later than 15 weeks.
The present TUNEL staining of the wrist and hand revealed that positivity was limited to the flexor digitorum profundus tendon and the associated lumbricalis muscles in the carpal tunnel. The suggested cell death in the lubricalis muscle seemed to correspond to the rearrangement of deep flexor tendons for each of the fingers [17]. Thus, if cell death occurs at the other sites in and around the carpal tunnel at 10-15 weeks, it may not be due to apoptosis but rather some other mechanism, such as that postulated by Castro-Obregón et al. [18]. Developmental cell death is mediated by multiple mechanisms and displays various morphologies [19, 20]: classical apoptosis involves DNA fragmentation, whereas other forms of programmed cell death do not.
Podoplanin immunoreactivity was seen in 2 of 4 specimens examined. Most of the positive lymphatic vessels were located in subcutaneous loose connective tissue, as described previously by our group [21]. Immunoreactivity for podoplanin was also demonstrated in connective tissues by Jin et al. [22]. However, the positive fibrous tissue and lymphatic vessels did not overlap with the area of CD68-positive macrophage accumulation. Although Melrose and Little [3] reported a crucial role of macrophages in lymphangiogenesis in the human fetus at 12 and 14 weeks of gestation (completely overlapping the developmental stage of the present materials), we found no or few CD68-positive macrophages in the areas where abundant lymphatic vessels were developing. Thus it seems unli kely that, in the human fetal extremities, macrophages transdifferentiate into endothelium of lymphatic vessels. Abe et al. [23] demonstrated CD34-positive fibrous tissue in human fetal extremities, but they were located on both the extensor and flexor sides of the joints. In addition, matrix substances (aggrecan, versican, hyaluronan, and tenascin-C) do not show a specific expression pattern that overlaps with areas of CD68-positive macrophage accumulation in the hand and foot (unpublished data), although Kjaer et al. [24] and Okita et al. [25] have demonstrated changes in the extracellular matrix around tendons depending on the degree of exercise.
Finally, it seems pertinent to address individual differences in the density or immunoreactivity of CD68-positive macrophages, even though our discussion is based on general observations. Although the clinical relevance of this study is still unclear, intrauterine ultrasound may be able to evaluate changes in muscle shape and volume at 15 weeks of gestation. Morphological differences in CD68-positive macrophages are likely related to specific conditions in utero, such as general inflammation, as well as the development of muscles and tendons.
Figures and Tables
Fig. 1
Foot and elbow of a 10-week fetus. (A, C) Hematoxylin and eosin staining, (B, D) CD68 immunohistochemistry. (B) and (D) are higher-magnification views of the squares in (A) and (C), respectively. CD68-positive macrophages are concentrated in the flexor aspects of the foot (B) and elbow (D). In the related muscles, the brachialis muscle contains particularly abundant positive cells. BR, brachioradialis muscle; C, cuneiform bones; CU, cuboid bone; FDL, tendon of the flexor digitorum longus muscle; FHL, tendon of the flexor hallucis longus muscle; MT, metatarsal bone. Scale bars=1 mm (A, C), 0.1 mm (B, D).
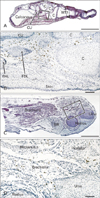
Fig. 2
Foot and hand of a 12-week fetus. (A, C, E) Hematoxylin and eosin staining, (B, D, F) CD68 immunohistochemistry, (G) TUNEL staining. (B, D, F) are higher-magnification views of the squares in (A, C, E), respectively. (G) corresponds to the square in (D). CD68-positive macrophages are concentrated in the flexor aspects of the foot (B, E) and hand (D). An especially high density of positive cells is evident in the loose connective tissue around the carpal tunnel (D). Stars in (D) indicate tendons of the flexor digitorum superficialis muscle. TUNEL-positive cells are seen in a tendon of the flexor digitorum profundus muscle (G). C, cuneiform bones; CU, cuboid bone; FDL, tendon of the flexor digitorum longus muscle; FHL, tendon of the flexor hallucis longus muscle; FPL, tendon of the flexor pollicis longus muscle; L, lumbricalis muscle; LPA, lateral plantar artery; MC, metacarpal bone; MT, metatarsal bone; NA, navicular; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling. Scale bars=1 mm (A, C, E), 0.1 mm (B, D, F). The magnification of panel G is the same as panel F.
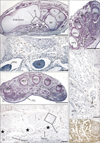
Fig. 3
Hand of a 12-week fetus. A specimen different from that shown in Fig. 2. D2-40 immunohistochemistry for lymphatic vessels (A) and CD68 immunohistochemistry (B, C). (B) and (C) are higher-magnification views of the squares B and C in (A), respectively. In (A), podoplanin or D2-40 is positive in lymphatic vessels (arrows), a fascia on the dorsal side of the carpal tunnel (stars) and cartilages. However, these positive sites do not correspond to sites of accumulation of CD68-positive macrophages (B, C). Scale bars=0.1 mm (A-C).

Fig. 4
Hand and elbow of a 15-week fetus. (A) Hematoxylin and eosin staining, (B-F) CD68 immunohistochemistry. (B-F) are higher-magnification views of the squares B, C, D, E and F in (A), respectively. CD68-positive macrophages are concentrated in the flexor aspect of the hand (A), along the abductor pollicis brevis muscle (Abd; C), along the pronator quadratus muscle (D), in the annular ligament of the radius (E) and in the cubital fossa (F). B tendon, tendon of the biceps brachii muscle; BR, brachioradialis muscle; FDP, a tendon of the flexor digitorum profundus; MC4, fifth metacarpal bone. Scale bars=1 mm (A), 0.1 mm (B). The magnification of panel C-F are the same as panel B.
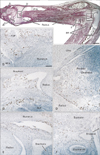
Fig. 5
Knee of a 15-week fetus and the calf of a 10-week fetus. (A-D) display a specimen different from that shown in Fig. 4, while (E) and (F) represent the same specimen as that in Fig. 1. (A, E) Hematoxylin and eosin staining, (B-D, F) CD68 immunohistochemistry. (B-D) are higher-magnification views of the squares B, C and D in (A), respectively. CD68-positive macrophages are concentrated in the flexor aspect of the knee, in contrast to the extensor aspect, where only osteoclasts are evident (C). (F) corresponding to the square in (E), shows CD68-positive macrophage accumulation in the medial, intermuscular connective tissue of the calf. MHG, tendon of the medial head of the gastrocnemius muscle; quad, quadriceps femoris (the most distal part); SM, tendon of the semimembranosus. Scale bars=1 mm (A, E), 0.1 mm (B-D, F).

Acknowledgements
This research was supported by Oral Health Science Center Grant hrc8 from Tokyo Dental College, and by a Project for Private Universities matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology), Japan, 2010-2012.
References
1. Benjamin M, McGonagle D. Basic concepts of enthesis biology and immunology. J Rheumatol Suppl. 2009. 83:12–13.
2. Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005. 115:2316–2319.
3. Melrose J, Little CB. Immunolocalization of lymphatic vessels in human fetal knee joint tissues. Connect Tissue Res. 2010. 51:289–305.
4. Ballard KJ, Holt SJ. Cytological and cytochemical studies on cell death and digestion in the foetal rat foot: the role of macrophages and hydrolytic enzymes. J Cell Sci. 1968. 3:245–262.
5. Garcia-Martinez V, Macias D, Gañan Y, Garcia-Lobo JM, Francia MV, Fernandez-Teran MA, Hurle JM. Internucleosomal DNA fragmentation and programmed cell death (apoptosis) in the interdigital tissue of the embryonic chick leg bud. J Cell Sci. 1993. 106(Pt 1):201–208.
6. Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994. 107(Pt 5):1159–1167.
7. Abood EA, Jones MM. Macrophages in developing mammalian skeletal muscle: evidence for muscle fibre death as a normal developmental event. Acta Anat (Basel). 1991. 140:201–212.
8. Fidziańska A, Goebel HH. Human ontogenesis. 3. Cell death in fetal muscle. Acta Neuropathol. 1991. 81:572–577.
9. McClearn D, Medville R, Noden D. Muscle cell death during the development of head and neck muscles in the chick embryo. Dev Dyn. 1995. 202:365–377.
10. Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973. 7(3):253–266.
11. Webb JN. The development of human skeletal muscle with particular reference to muscle cell death. J Pathol. 1972. 106:221–228.
12. Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999. 154:385–394.
13. Abe S, Rhee SK, Osonoi M, Nakamura T, Cho BH, Murakami G, Ide Y. Expression of intermediate filaments at muscle insertions in human fetuses. J Anat. 2010. 217:167–173.
14. Sbernardori MC, Fenu G, Pirino A, Fabbriciani C, Montella A. Histogenesis and morphology of the flexor tendon pulley system in the human embryonic hand. J Hand Surg Br. 2000. 25:175–179.
15. Rowshan K, Hadley S, Pham K, Caiozzo V, Lee TQ, Gupta R. Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology. J Bone Joint Surg Am. 2010. 92:2270–2278.
16. Takahashi M, Ward SR, Marchuk LL, Frank CB, Lieber RL. Asynchronous muscle and tendon adaptation after surgical tensioning procedures. J Bone Joint Surg Am. 2010. 92:664–674.
17. Cho KH, Kim JH, Ha YS, Murakami G, Cho BH, Abe S. Development of the deep flexor tendons and lumbricalis muscle in the hand and foot: a histological study using human mid-term foetuses. Folia Morphol (Warsz). 2012. 71:154–163.
18. Castro-Obregón S, Del Rio G, Chen SF, Swanson RA, Frankowski H, Rao RV, Stoka V, Vesce S, Nicholls DG, Bredesen DE. A ligand-receptor pair that triggers a non-apoptotic form of programmed cell death. Cell Death Differ. 2002. 9:807–817.
19. Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl). 1990. 181:195–213.
20. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995. 146:3–15.
21. Jin ZW, Nakamura T, Yu HC, Kimura W, Murakami G, Cho BH. Fetal anatomy of peripheral lymphatic vessels: a D2-40 immunohistochemical study using an 18-week human fetus (CRL 155 mm). J Anat. 2010. 216:671–682.
22. Jin ZW, Song KJ, Lee NH, Nakamura T, Fujimiya M, Murakami G, Cho BH. Contribution of the anterior longitudinal ligament to ossification and growth of the vertebral body: an immunohistochemical study using the human fetal lumbar vertebrae. Surg Radiol Anat. 2011. 33:11–18.
23. Abe S, Suzuki M, Cho KH, Murakami G, Cho BH, Ide Y. CD34-positive developing vessels and other structures in human fetuses: an immunohistochemical study. Surg Radiol Anat. 2011. 33:919–927.
24. Kjaer M, Magnusson P, Krogsgaard M, Boysen Møller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006. 208:445–450.
25. Okita M, Yoshimura T, Nakano J, Motomura M, Eguchi K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J Muscle Res Cell Motil. 2004. 25:159–166.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download