Abstract
In rats, ageing results in dysfunctional patterns of micturition and diminished sexual reflexes that may reflect degenerative changes within spinal circuitry. In both sexes the dorsal lateral nucleus and the spinal nucleus of the bulbospongiosus, which lie in the L5-S1 spinal segments, contain motor neurons that innervate perineal muscles, and the external anal and urethral sphincters. Neurons in the sacral parasympathetic nucleus of these segments provide autonomic control of the bladder, cervix and penis and other lower urinary tract structures. Interneurons in the dorsal gray commissure and dorsal horn have also been implicated in lower urinary tract function. This study investigates the cellular localisation of PG-21 androgen receptors, steroid receptor co-activator one (SRC-1) and the phosphorylated form of c-AMP response element binding protein (pCREB) within these spinal nuclei. These are components of signalling pathways that mediate cellular responses to steroid hormones and neurotrophins. Nuclear expression of PG-21 androgen receptors, SRC-1 and pCREB in young and aged rats was quantified using immunohistochemistry. There was a reduction in the number of spinal neurons expressing these molecules in the aged males while in aged females, SRC-1 and pCREB expression was largely unchanged. This suggests that the observed age-related changes may be linked to declining testosterone levels. Acute testosterone therapy restored expression of PG-21 androgen receptor in aged and orchidectomised male rats, however levels of re-expression varied within different nuclei suggesting a more prolonged period of hormone replacement may be required for full restoration.
The rat lumbosacral cord contains many neurons involved in the spinal circuitry that modulates lower urinary tract (LUT) function. The sacral parasympathetic nucleus (SPN), which lies in spinal segments L6-S1, contains neurons that project to the parasympathetic component of the major pelvic ganglion in male rats [1-6] and the paracervical ganglion in females [7, 8]. The postganglionic parasympathetic neurons of these ganglia innervate the cervix, clitoris, bladder, penis, prostate, rectum, uterus, vagina, and vas deferens [9-14]. A number of studies have suggested that the SPN may also contain interneurons that act in concert with the parasympathetic preganglionic neurons to control bladder [15-17], and genital function, e.g., urethrogenital [18, 19] and penile reflexes [20-22]. These studies also identified interneurons that appear to be integral to the circuitry subserving bladder and genital functions in the dorsal grey commissure (DGC) and the superficial laminae of the dorsal horn (DH) [23-25] both of which receive sensory afferent projections from the pelvic viscera [3].
The L6-S1 region of the rat spinal cord contains a number of motor nuclei, some of which supply the muscles of the pelvic floor. The axons of motor neurons belonging to the dorsolateral nucleus (DLN) project via the pudendal nerve to the ischiocavernosus muscles and the external urethral sphincter [26-28]. The spinal nucleus of the bulbospongiosus (SNB), contains motor neurons that innervate the levator ani muscle (described in some studies as the dorsal bulbospongiosus), the ventral bulbospongiosus muscles and the external anal sphincter [27, 28]. Motor neurons of the DLN/SNB play a key role in coordinating perineal muscle activity associated with penile erection [25] and maintaining faecal [29] and urinary continence [24, 30]. They are highly androgen sensitive throughout life. During development, the normally higher levels of circulating androgens in males prevent apoptosis of DLN/SNB motor neurons in male rats [31, 32] and as a consequence these nuclei contain significantly greater numbers of motor neurons in adult males than females [26, 33-35]. Segments L5-L6 also contain the retrodorsolateral nucleus (RDLN), which innervates the flexor digitorum brevis muscle of the rat foot [36, 37] and a component of the ventral medial nucleus (VMN) but neither is sexually dimorphic or significantly androgen dependent [26, 35, 36].
The effects of androgens on perineal motor neurons persists into adulthood as reductions in circulating testosterone levels can cause significant changes to their morphology. Orchidectomy has been shown to induce a decrease in the number and size of DLN/SNB motor neuron somata [34, 38, 39] as well as reductions in their total dendritic length and dendritic branching [40, 41]. Ageing has also been reported to be correlated with reductions in soma size, dendritic length and of the abundance of synaptic inputs [42-44]. This may be a consequence of the declining androgen levels observed in aged male rats [45-48] which are accompanied by a reduction in the expression of the androgen receptor PG-21 in SNB motor neurons [46, 47]. It is well established that nuclear steroid hormone receptors (including androgen receptors) bind to specific DNA hormone response elements that promote the transcription of steroid (e.g., androgen) responsive genes to up-regulate cellular activity [49, 50]. Binding of steroid receptors to the promoter region of the target gene is enhanced by a number co-activators including steroid receptor co-activator one (SRC-1) and c-AMP response element binding protein (CREB) [51-53]. CBP is a CREB-binding protein which acts as a co-activator for the transcription factor CREB [54]. CREB is activated by phosphorylation of a serine residue at position 133 by a c-AMP dependant protein kinase [55, 56], and this stimulates the transcription of c-AMP responsive genes by binding to the c-AMP response element [57]. This transcriptional activity of phosphorylated CREB (pCREB) has been linked to the neuronal cell signalling cascades induced by neurotrophin binding [58, 59] and promotes neuronal survival and plasticity during aging [60-62]. Consequently, any decline in the activity or expression of pCREB may lead to cellular dysfunctions that are synergistic to the age-related degenerative changes observed in perineal motor neurons as a consequence of androgen depletion. Previous studies have used immunhistochemistry to determine the location of cells in lumbosacral cord that express nuclear immunoreactivity to PG-21 [46, 63-68], SRC-1 [66, 69-71], and pCREB [72]. However previous studies of the effects of ageing on the expression of these proteins have been confined mainly to the SNB of male rats [46, 47, 66, 72]. A preliminary study by Ranson et al. [71], though wider in scope, did not analyse sufficient numbers of animals to obtain statistically significant results. The object of the current investigation was to provide a comprehensive and quantitative description of the cellular expression of PG-21 in male rats and SRC-1/pCREB in both sexes, within functionally defined regions (DGC, DH, DLN, RDLN, SNB, SPN, and VMN) of the lumbosacral cord. The pattern of immunoreactivity in young (3 months) and aged (24 months) animals was compared. In addition the effect of orchidectomy, with or without subsequent testosterone replacement, was examined on the cellular expression of these receptors and associated proteins in young and aged male rats.
Male and female Wistar rats (obtained from Harlan UK Ltd., Bicester, Oxon, UK) from two age groups of between 12-15 weeks (33 young males and 8 young females) and 104-112 weeks (18 aged males and 8 aged females), were used in the study. The stage of the oestrous cycle of the young females was not known at the time of anaesthetisation. The aged rats were taken from an established aged colony within Cardiff University. Animals were maintained under the conditions of a 12 hour light/dark cycle, constant temperature and humidity, with ad libitum watering and feeding. All procedures performed were in accordance with UK. Home Office regulations under the Animals (Scientific Procedures) Act 1986. Experiments were designed to reduce the number of animals required and to minimize animal discomfort through the use of deep anaesthesia during surgery and by the administration of post-operative analgesics.
Male Rats (20 young, 10 aged) were bilaterally orchidectomised under Halothane anaesthesia (4% in O2, Concord Pharmaceuticals Ltd., Dunmow, Essex, UK). After surgery the animals were administered the analgesic buprenorphine (1% by volume, Vetergesic, Reckitt Benckiser Healthcare [UK] Ltd., Hull, UK) in their drinking water. After a recovery period of five days, seven of the young rats were processed for immunohistochemistry without having undergone any form of testosterone replacement schedule. The remaining animals each received a single subcutaneous injection containing 500 µg of testosterone propionate (TP; T-1875), dissolved in sesame oil (S-3547) both from Sigma-Aldrich Co. Ltd. (Poole, Dorset, UK). After a period of 2 hours spinal segments from these animals were also processed for immunohistochemistry. All procedures were approved and carried out under the Animals (Scientific Procedures) Act 1986, UK.
All animals were terminally anaesthetized using 'Euthatal' (sodium pentobarbitone at 400 mg/kg i.p., J. M. Loveridge P.L.C., Southampton, UK) and transcardially perfused with 125 ml of 0.9% saline solution followed by 300-500 ml of fixative (4% paraformaldehyde in 100 mM sodium phosphate buffer, pH 7.4). Spinal segments L5-S1 were removed and fixed for a further 3 hours by immersion in the same solution then stored overnight at 4℃ in 100 mM sodium phosphate buffer containing 30% sucrose. Spinal segments were then sectioned at 45 µm using a Bright OTF5000 Cryostat (Bright Instrument Co. Ltd., Huntingdon, Cambridgeshire, UK). Sections were viewed under a light microscope to ensure collection of those containing the SPN at S1 levels as well as the entire caudal-rostral extent of the DLN/SNB (Fig. 1). Sequential sections were placed in phosphate buffered saline (PBS) within three separate vials, one for each primary antibody. This ensured that sections stained with a particular antibody were separated by at least 90 µm and that cells demonstrating nuclear immunoreactivity could not be counted twice in the subsequent analysis. The sections were then washed in buffer and treated with 0.3% hydrogen peroxide in PBS to quench any endogenous tissue peroxidases. Further washing was followed by immersion for 30 minutes in a permeabilising/blocking solution consisting of 10% normal goat serum (cat no. 005-000-121, Stratech Scientific, Soham, Cambridgeshire, UK) in PBS containing 0.3% Triton X-100 (T9284, Sigma-Aldrich Co. Ltd.). They were then incubated for 3 days at 4℃ in the relevant primary antibody in PBS containing 1% normal goat serum and 0.3% Triton X-100. The primary antibodies were 1) rabbit anti-PG-21 (1 : 333, androgen receptor, cat no. 06-680, Upstate/Chemicon Europe Ltd., Chandlers Ford, Hampshire, UK)-raised against a synthetic peptide representing amino acids 1-21 of human androgen receptor; 2) rabbit anti-SRC-1 (1 : 333, Sc-8995, Autogen Bioclear, Calne, Wiltshire, UK), a rabbit polyclonal antibody raised against amino acids 350-690 of SRC-1 of human origin; and 3) rabbit anti-pCREB (1 : 500, cat no. 06-519, Upstate/Chemicon Europe Ltd.), a polyclonal antibody raised against a synthetic phosphopeptide corresponding to residues 123-136 of rat CREB. Following the primary antibody incubation, sections were washed in PBS then reacted with a Vector Elite ABC-peroxidase kit (PK-6101, Vector Laboratories Ltd., Peterborough, UK) involving a 2 hour incubation in anti-rabbit IgG solution followed by 1 hour in avidin-biotin-peroxidase complex. After washing in PBS, sections were developed with a VIP substrate kit (SK-4600, Vector Laboratories Ltd.) or DAB (SK-4100, Vector Laboratories Ltd.) using identical incubation times. Sections were mounted on glass slides, cleared through an alcohol/xylene series and coverslipped in DPX mounting medium (R1340, Agar Scientific Ltd., Essex, UK).
The labelled sections were divided into seven groups comprising non-orchidectomised young males, non-orchidectomised aged males, orchidectomised young males, orchidectomised young males+TP, orchidectomised aged males+TP, young females and aged females. Sections were viewed under bright field illumination with a Leica DMR microscope (Leica Microsystems [UK] Ltd., Milton Keynes, Bucks, UK) and representative images were captured with a SPOT digital camera (Diagnostic Instruments Inc, Sterling Heights, MI, USA). Selected images showing the distribution of immunoreactivity for pCREB, PG-21 or SRC-1 were subsequently compiled into figures using Adobe Photoshop 6 (Adobe Systems Inc., San Jose, CA, USA). Small changes to the brightness and contrast levels of these images were made in order to optimise the visibility of the immunolabelling for documentation purposes but no processing was carried out on images used to obtain the quantitative data described below.
Sections that were undamaged, i.e., with no folding or tearing within the counting areas (Fig. 1), were used for quantitative analyses. Counts were made on sections, viewed with a ×40 objective lens, of the numbers of nuclei displaying immunoreactivity to pCREB, PG-21 or SRC-1 within the selected regions of the lumbosacral spinal cord (Fig. 1). To achieve this, a graticule square covering 240×240 µm was used for RDLN, while one of 180×180 µm was used for DGC, DLN, SNB, SPN, VMN and of 120×120 µm for the DH. Only immunoreactive somata lying entirely within the confines of the square and displaying medium to high-density nuclear staining were counted. Lightly stained cells were omitted from the count as it was our experience that the antibody staining was very sensitive to minor changes in fixation which sometimes increased the number of such cells, suggesting that they represented false positives. All counts were made by a single observer to ensure consistency of evaluation. Cell counts were taken from all of the selected regions, where present (e.g., RDLN neurons are not found at S1 levels) in two sections at L5, four at L6 and two at S1. In this fashion, 48 sample counts (eight per animal/one per section) were made from the DGC and DH whilst 36 sample counts (six per animal/one per section) were made for the DLN, RDLN, SNB, SPN for each of the antigens studied. In the case of the VMN, which is primarily found at L6, a smaller sample size of 24 samples was used (four per animal/one per section). For DLN, RDLN, SNB, and VMN, only those cells that likely represented motor neurons (i.e., with a cell diameter greater than 25 µm) were counted. For the SPN a distinction was made between immunoreactive cells with a diameter of 20-25 µm which probably represent preganglionic neurons, and those that were smaller and likely to be interneurons or glial cells.
For each antibody and each region of analysis e.g., DGC (through L5-S1), data sets were analysed for homogeneity of variance using Bartlett's test. Since at least one of our data sets demonstrated heterogeneity, we used unpaired t-tests with Welch's correction (which does not assume equal variances) in order to compare the data from young and aged animals from each region in both sexes. In addition further analyses were performed to determine the effects of orchidectomy and/or TP replacement. The means and standard errors described below and displayed in the bar charts 7 represent the values obtained from all the data collected for a particular region at each age/experimental manipulation. Thus e.g., for the DLN, the mean cell number was obtained from 120 images in total and does not represent the mean of means for each individual rat. A probability of less than or equal to 0.05 was considered statistically significant in all analyses and all references to significant difference in the results section equate to this value.
Cells displaying immunoreactivity for PG-21 in non-orchidectomised rats (Fig. 2A) were found to be most prominent in the pudendal motor nuclei (DLN/SNB) and the DGC. In addition both the DH and SPN contained numerous immunoreactive cells, whilst within the RDLN there were relatively few cells with only low levels of expression of PG-21. Within the SNB of non-orchidectomised rats PG-21 immunoreactivity was present in the nuclei of cells which on the basis of size appeared to be motor neurons (Fig. 2B). Sections through this nucleus contained of 2.91±0.3 (mean±SEM) cells immunoreactive for PG-21 (Fig. 3). Orchidectomy completely abolished this nuclear staining (not shown) but nuclear expression of PG-21 immunoreactivity was restored by TP treatment (Fig. 2C) to a 2.3±0.2 motor neurons per section (Fig. 3); a figure not significantly different to that of young non-orchidectomised rats. By contrast, in aged animals 1.75±0.21 cells per section showed nuclear immunoreactivity for PG-21 (Fig. 3) in this nucleus (Fig. 2D), which represented approximately a 40% decline compared to young non-orchidectomised rats (significant at P<0.05). In orchidectomised aged rats TP treatment also resulted in re-expression of nuclear PG-21 (Fig. 2E) in 2.08±0.23 cells per section which was not significantly different to the number of cells observed for aged non-orchidectomised rats but was significantly lower (P<0.05) than that seen in young non-orchidectomised rats. In the DLN, nuclear expression of PG-21 was also present in putative motor neurons of non-orchidectomised animals (Figs. 2F, 3) in 4.89±0.41 immunopositive cells per section. This was completely abolished by orchidectomy leaving only residual cytoplasmic labelling in some cells (Fig. 2G). TP treatment of orchidectomised young animals was significantly less effective in stimulating nuclear re-expression of PG-21 within DLN motor neurons (Fig. 2H) generating a mean of 3.05±0.3 immunopositive cells per section. Likewise ageing also significantly (P<0.05) reduced the numbers of PG-21 expressing cells in the DLN to 3.81±0.37 (Figs. 2I, 3) which represented a decline of approximately 23% from the numbers seen in young, non-orchidectomised rats. In the DLN, TP treatment of orchidectomised aged rats generated levels of nuclear PG-21 that were not significantly different from non-orchidectomised aged rats but remained well below those of young non-orchidectomised animals. In regions other than the pudendal nuclei, the effects of ageing and/or testosterone replacement were similar to those described for the DLN. In the case of the DGC the highest numbers of cells expressing PG-21 immunoreactivity were seen in sections obtained from young non-orchidectomised rats (Fig. 2J) with a mean of 41.8±1.36 labelled cells per 180×180 µm quadrat (Fig. 3). This was in marked contrast to orchidectomised young rats receiving TP treatment in which the population of PG-21 immunoreactive cells (19.94±1.28) was only 48% of this value (Figs. 2K, 3). Similarly, in aged non-orchidectomised rats, the number of PG-21 expressing neurons in this region (30.3±1.29, representing a 28% reduction), was significantly lower (P<0.05) than in young (non-orchidectomised) animals (Fig. 2L). In contrast to the young animals, TP treatment in aged orchidectomised rats restored PG-21 re-expression to a level that did not significantly differ from aged intact animals though it remained well below young levels (Fig. 3). In the DH, PG-21 immunolabelled cells are largely confined to laminae I-III (Fig. 2M). Averaged data from a 120×120 µm quadrat placed in the middle of the DH revealed that young non-orchidectomised rats showed the greatest abundance of PG-21 immunoreactive cells (26.7±1.5) (Fig. 3). The DH of testosterone treated orchidectomised young animals and non-orchidectomised aged rats contained 15.65±0.83 and 20.17±1.12 cells per section, respectively. Consequently testosterone replacement in young animals resulted in re-expression of nuclear PG-21 in approximately 59% of the number of DH cells seen in young intact animals or 81% of the number in aged animals. On the other hand, TP treatment in aged orchidectomised rats produced comparable levels of expression of PG-21 to those seen in intact aged males. In the SPN (Fig. 2N) two types of cell were analysed. Large immunoreactive nuclei (10-12 µm) are likely to belong to preganglionic parasympathetic neurons, whilst smaller (5-7 µm) nuclei probably belong to glial cells or interneurons. In line with previous observations, analysis of this region revealed that PG-21 immunoreactive cells were most abundant in young (non-orchidectomised) rats and that ageing reduced their number by approximately 24% for probable preganglionic neurons and 26% for other cell types (Fig. 3). A comparable situation was found in young orchidectomised rats receiving TP injections, in which PG-21 re-expression occurred in about 40% of preganglionic neurons and 43% of glial cells/interneurons compared to young intact animals. TP treatment in aged animals was again more effective in inducing nuclear re-expression of PG-21 in aged orchidectomised rats (Fig. 3) although young (non-orchidectomised) levels of expression were never achieved.
Cells immunoreactive for SRC-1 were extensively distributed throughout the grey matter of the spinal cord (Fig. 4A). Within the SNB of male rats (Fig. 4B-E) larger cells, (putative motor neurons), stood out distinctively from the smaller cells surrounding them (Fig. 4C). Within the SNB, the highest numbers of putative motor neurons expressing SRC-1 were observed in young male rats (Fig. 4B-D) and neither the effects of orchidectomy (Fig. 4C) nor of orchidectomy with testosterone replacement (Fig. 4D) produced numbers significantly different from those seen in non-orchidectomised young male rats (Fig. 5). However in aged intact rats (Fig. 4E) there was approximately an 18% reduction (Fig. 5) in the numbers of putative motor neurons compared to young intact rats (3.67±0.3 young vs. 3.00±0.28 aged, significant at P<0.05). In female rats (not shown) a maximum of 1-2 of putative motor neurons, were occasionally observed in the region of the SNB. In the DLN the major significant difference observed in male rats was between young (Fig. 4F) and aged (Fig. 4G) non-orchidectomised animals. In the aged animals there was an approximately 23% reduction (Fig. 5) in the numbers of putative motor neurons expressing nuclear SRC-1 (6.75±0.54 young vs. 5.22±0.42 aged, significant at P<0.05). In the DLN of female rats (Fig. 4H, I) the numbers of SRC-1 expressing putative motor neurons was not significantly affected by age, being 3.56±0.33 for young, and 3.42±0.24 for aged animals (Fig. 5). By contrast the number of motor neurons within the DLN of females was significantly less (P<0.05) than that observed in either young or aged males (Fig. 5). Nuclear expression of SRC-1 was also observed in cells of the RDLN (Fig. 4J, K) and VMN (Fig. 4L). The greatest difference in cell numbers was seen between intact male rats (Fig. 4J, K), in which there was an age-related decline of 16% in neurons exhibiting nuclear expression of SRC-1 (Fig. 5). In female rats RDLN cell number did not change significantly with age and was similar to that in male rats (Fig. 5). In the VMN there were no significant differences in labelled cell numbers with age, experimental procedure or gender (Fig. 5). In the DH considerable labelling was seen in laminae I-III of both males (Fig. 4M) and females (not depicted). Fourteen percent fewer SRC-1 immunoreactive cells were seen in the DH of young female rats compared to young intact males (Fig. 5). TP treatment appeared to increase the expression of SRC-1 in orchidectomised rats by approximately 19% compared to intact rats of the same age, whilst in aged rats the number of cells expressing SRC-1 appeared to decline by 14% from the levels seen in young animals (44.4±1.6 cells in young vs. 38.1±1.14 in aged, significant at P<0.05) (Fig. 5). By contrast the numbers of cells with nuclei expressing SRC-1 in the DH of female rats did not change with age (Fig. 5). In the DGC (Fig. 4N) the process of orchidectomy (with or without TP therapy) appeared to increase the number of cells expressing SRC-1. For example, in the DGC of young orchidectomised males, an average of 53.0±1.9 cells expressed SRC-1 per 180×180 µm quadrat, compared to 44.42±1.6 in young intact rats. The value for young intact rats was significantly (P<0.05) higher than that obtained for aged intact rats (38.1±1.12 cells, a decline of approximately 14%). In females, the number of SRC-1 expressing cells was not significantly different between young and aged animals but was again significantly lower than in young adult males (Fig. 5). In the SPN (Fig. 4O), it was again possible to distinguish different phenotypes of SRC-1 expressing cells (Fig. 4P). The numbers of putative preganglionic SRC-1 expressing cells in the SPN were significantly higher in males than in females (7.31±0.5 cells for young males vs. 5.00±0.5 cells for young females) (P<0.05) however there was no significant age-related change in either sex (Fig. 5). As in the DH, orchidectomy with TP treatment appeared to increase the number of putative preganglionic neurons expressing SRC-1 by approximately 17% (Fig. 5). A similar relationship was seen for the putative glial cell/interneuron population, with a significant increase in expression of 22% following orchidectomy and of 11% for orchidectomised animals receiving TP (Fig. 5). However aged animals displayed a reduction of around 15% in the number of smaller (glial/interneuron) cells located within the SPN compared to young non-orchidectomised males. This age-related difference was not seen in female rats (Fig. 5). As for the potential preganglionic neurones within this nucleus, the larger cells that expressed SRC-1 were 16% more numerous in young intact males than in young females (Fig. 5).
In lumbosacral spinal cord, cells whose nuclei express pCREB were abundant throughout the grey matter and were often also observed in the white matter (Fig. 6A). Within the SNB, putative motor neurons immunoreactive for pCREB were surrounded by smaller cells, probably glia or interneurons that were also immunoreactive (Fig. 6B, C). In young intact male rats, one to ten (4.00±0.41) putative motor neurons immunoreactive for pCREB were observed per section (Figs. 6B, 7). The abundance of labelled motor neurons in the SNB of young intact male rats (Fig. 6C) or orchidectomised, TP treated rats (Fig. 6D) was not significantly different (Fig. 7). Intact aged rats (Fig. 6E) displayed a 47% decrease (2.14±0.25, P<0.05) in pCREB immunoreactive motor neurons per section compared to young intact males (Fig. 7). As with SRC-1, very few putative motor neurons immunoreactive for pCREB were present in the SNB of female rats (Fig. 7). The DLN exhibited a similar pattern of putative motor neurons immunoreactive for pCREB surrounded by smaller labelled cells (Figs. 6F-I). In young intact males (Fig. 6F) up to fifteen pCREB immunoreactive motor neurons were present in each section through the DLN (mean, 6.17±0.43) (Fig. 7). This value was significantly higher than that recorded in either aged males (Fig. 6G) or in young or old female rats (Figs. 6H, I, 7). The number of immunoreactive motor neurons seen in aged males (3.97±0.04) represented a 36% decline compared to young adult males. The number of pCREB immunoreactive motor neurons in the DLN of females is approximately 56% of that of young males (Fig. 7). There was no significant age-related change in the DLN of female rats (Figs. 6H, I, 7). In the RDLN (Fig. 6J), putative motor neurons expressing pCREB were again surrounded by numerous smaller immunoreactive cells. In intact aged male rats, there was a decline of 25% in pCREB immunoreactive motor neurones in this nucleus. pCREB immunoreactive cells were also present in the VMN (not shown). In this nucleus, age-related changes in numbers were seen in female animals in which there was a decline from 3.3±0.5 cells to 1.5±0.39 cells per section (P<0.05). In terms of pCREB expression there was no sexual dimorphism in either the RDLN or VMN. Cells expressing pCREB nuclear immunoreactivity were also abundant in the SPN (both large and small) (Fig. 6K), the DH (Fig. 6L, M) and the DGC (Fig. 6N, O). In the SPN, there was no age-related difference in the numbers of probable preganglionic or other cell types in either intact male or female rats though there was a small but significant difference between young female and young intact male rats (10.44±0.65 female vs. 7.7±0.55 male, significant at P<0.05). This difference was not however, seen in the population of small pCREB immunoreactive cells that probably represent glia or interneurons. In the SPN of young males there appeared to be a small increase in the number of pCREB expressing cells of all types following orchidectomy (with or without TP treatment) (Fig. 7). This was also seen in both the DH and the DGC. In the DH, cells expressing pCREB nuclear immunoreactivity were densely distributed throughout the first three laminae (Fig. 6L, M). Analysis of 120×120 µm quadrats in these laminae (Fig. 7) revealed an average of 39.42±1.13 pCREB immunoreactive cells in young intact males (Fig. 6L) compared to 49.27±1.64 cells in young orchidectomised males (Fig. 6M) and 51.52±1.54 in young male orchidectomised rats treated with TP (not shown). There were significant differences in numbers (P<0.05) between orchidectomised and young intact males. The addition of TP did not significantly affect numbers in young orchidectomised animals. Female rats exhibited significantly higher numbers of pCREB immunoreactive cells in DH than males (Fig. 7). The effects of ageing differed between the sexes: while there was no significant change with age between the numbers of pCREB expressing cells in the DH of male rats, there was an age-related decline of about 10% in females. Many of the changes described for the DH were also seen in the DGC (Fig. 6N, O). Orchidectomy, with or without TP administration, resulted in increased numbers of pCREB immunoreactive cells in both young and aged males (Fig. 7). Young and aged female rats had higher numbers of pCREB immunoreactive cells than intact males of equivalent age (Fig. 7). However in contrast to the DH, though immunoreactivity for pCREB in the DGC did not differ significantly with age in female rats, there was an 18% decline in intact males rats (Fig. 7).
This study has shown that the nuclear expression of PG-21, SRC-1, and pCREB is widespread within the spinal nuclei associated with the control of LUT functions. Ageing resulted in a decrease in the number of cells immunoreactive for these proteins, within the DH, DGC, SPN, and pudendal motor nuclei that are involved in the control of micturition and/or sexual function. However these reductions were confined primarily to male rats. This suggests that the decreases in expression are probably linked to testosterone levels (which decline in aged male rats) and could contribute to the sexual dysfunction observed in aged male rats. The time of onset of these changes could not be determined by the availability of only 'young adult' and 'aged' groups of animals. Acute testosterone replacement therapy in orchidectomised rats did not return the numbers of cells expressing nuclear PG-21 to levels seen in intact animals in either age group, suggesting that a more prolonged replacement schedule is required for full restoration. Studies of SRC-1 and pCREB expression following orchidectomy with or without testosterone replacement produced inconsistent patterns of labelling which likely reflects the fact that these proteins can be activated through a variety of different signalling pathways.
In the present study, immunohistochemistry for the androgen receptor PG-21 produced nuclear labelling in significant populations of cells within the nuclei associated with control of the LUT. The DLN/SNB, which constitute the pudendal motor nuclei, contained many strongly labelled motor neurons while in the RDLN only low levels of immunoreactivity were observed. This is in agreement with many previous studies [46, 47, 63-65, 73]. The current investigation showed that cells with nuclear PG-21 expression were also abundant in the DGC and SPN and to a lesser extent within the DH of L6-S1 cord. PG-21 expression within other spinal nuclei has only been briefly described previously [46, 68, 73] in studies that considered neither the effects of ageing nor of orchidectomy with hormone replacement.
The distribution of PG-21 immunoreactive cells matches that of populations within L6-S1 revealed by viral tracer injections into the bladder, penis and perineal muscles [16, 20, 74, 75]. Furthermore, male rat urethrogential reflexes [21] or mating behaviour [73] leads to immediate early gene c-fos expression predominantly in the DGC and SPN. Ninety-nine percent of the c-fos expression induced by mating was found in cells immunoreactive for androgen receptors [73]. Retrogradely labelled parasympathetic neurons projecting to the major pelvic ganglion have been shown to express PG-21-immunoreactivity which was diminished by orchidectomy [68]. These observations suggest that the PG-21 nuclear expressing cells seen in the current study are likely to be involved in both male sexual reflexes and micturition. Functionally, however, orchidectomy does not appear to alter natural micturition cycles in rats [76]. Motor neurons innervating the external anal and urethral sphincters, despite displaying PG-21 immunoreactivity [63], appear insensitive to the effects of androgen and do not exhibit morphological changes in response to orchidectomy, with or without testosterone replacement [77]. This suggests that the effect of circulating androgens on the lumbosacral cord is largely confined to the circuitry controlling male sexual function. It is well established that a decline in the levels of circulating androgen in male rats, either through orchidectomy or the process of ageing, has a strong negative effect on copulatory behaviour and penile reflexes [78-83]. This can be correlated with a decline in the nuclear expression of steroid receptors, including PG-21 [46, 47, 67], which has a direct effect on the structural and physiological properties of the cells concerned [49, 50]. Studies by Matsumoto and Prins [46, 47] have revealed that orchidectomy completely abolishes the nuclear expression of PG-21 in SNB motor neurones and that ageing leads to an approximately 41% reduction in the numbers of SNB motor neurons displaying nuclear PG-21 immunoreactivity, a figure almost identical to the one we report here (40%). We can also confirm the complete abolition of nuclear PG-21 expression in all cells of the DLN at L5-S1 following orchidectomy. However, we also observed reductions in PG-21 nuclear expressing cells in the other nuclei associated with male sexual function, i.e., DGC (by 28%), DH (19%), DLN (23%), DH (19%), and SPN (24% for probable preganglionic and 26% for other cell types).
In order to reverse the age-related decline [47, 67] we injected rats with 500 mg of TP, five days after orchidectomy. Two hours after this hormone replacement therapy, PG-21 expression in SNB motor neurons either matched or surpassed that seen in intact animals. To facilitate comparison with Masumoto and Prins' work [46, 47], we employed the same treatment regime and we were able to confirm their results for the SNB, however our observations for the other spinal nuclei associated with male sexual function were less consistent. In young male rats, TP replacement did not lead to significant re-expression in any of the other nuclei assessed (DGC, DH, DLN or SPN). In aged rats, TP administration was more effective in inducing nuclear re-expression of PG-21 although it was not sufficient to restore the levels to those of gonadally intact young males. Consequently therapies aimed at restoring sexual function in aged animals must recognise that testosterone levels are already declining at eleven months of age [48] and that this fall is accompanied by a shrinkage of SNB motor neuron dendrites [42]. By 22 months, the dendrites are 50% smaller than at 9 months [42] but this decline can be reversed by the implantation of silastic capsules containing testosterone for a period of six weeks at an age of 20.5 months. This treatment regime may be more effective in restoring PG-21 expression in the nuclei of cells controlling sexual function than the one employed here, however whether testosterone replacement therapy alone is sufficient to restore full copulatory behaviour in aged males remains uncertain. It has been shown that long term testosterone replacement (e.g., for up to 6 months from age 18 months) does not restore the number of mounts and ejaculation episodes to levels seen in young gonadally intact animals [78, 79, 84]. This suggests that low serum testosterone levels are only one factor contributing to neurally mediated age-related sexual dysfunction observed in male rats. Other factors such as changes in the synaptic inputs to the L6-S1 nuclei from both intraspinal and supraspinal sources, may be equally as important. Indeed studies from our laboratory have demonstrated that glutamatergic synapses on the dendrites of SNB motor neurones decrease significantly with age [44]. Likewise there is a decline in descending serotonergic pathways terminating in the DGC and DLN and also in the abundance of axon terminals immunoreactive for substance P or tyrosine hydroxylase, within the DGC and SPN [70, 85].
As steroid receptor co-activators enhance transcription of steroid responsive genes [52, 53] any decline in PG-21 expression as a consequence of ageing or orchidectomy is likely to be reflected in changes in the expression of SRC-1. In male rats, SRC-1 nuclear expression was seen in all populations of cells that demonstrated PG-21 immunoreactivity, although the number of SRC-1 immunoreactive cells was markedly more abundant in the DH. Nuclear expression of SRC-1 was also seen in motor neurons of the RDLN and VMN of males which exhibit scant or no PG-21 expression. In female rats, SRC-1 was present in all of the lumbosacral spinal nuclei associated with micturition or sexual function as well as the RDLN and VMN. This has been reported previously for both males and females [71], however statistical analysis in the present investigation has generated new findings. Our previous subjective conclusion that female rats exhibited age-related changes in the numbers of cells expressing nuclear SRC-1 was not confirmed for any of the spinal nuclei assessed. In the light of the observations presented here, the single 24 month old female available for analysis in the previous study appeared to exhibit unusually low levels of SRC-1 expression. The current investigation also revealed that the number of cells with nuclear expressed SRC-1 was significantly lower in females than in males for many of the nuclei analysed (reductions were 17% in DGC, 14% in DH, 47% in DLN, and in the SPN, 31% for putative preganglionic neurons and 6.6% for other cell types). The SNB is virtually absent in females and there was no sex difference for the RDLN and VMN. The sexual dimorphism probably reflects the developmental consequences of lower levels of circulating testosterone in females [26, 31-35].
In the rat spinal cord, the distribution of SRC-1 immunoreactivity is probably correlated with the expression of type 1 nuclear receptors which bind androgens, estrogens, glucocorticoids and progesterone [51]. In female rats and cats, estrogen receptor immunoreactivity has been identified in the nuclei of cells within laminae I/II of the DH, and in the DGC and SPN of the lumbosacral cord [86-89]. By combining retrograde tracing with estrogen receptor localisation, it has been shown in female cats that SPN neurons projecting to the bladder express estrogen immunoreactivity [86]. Vaginocervical mechanostimulation induces virtually identical patterns of c-fos and estrogen receptor expression in the DH and SPN [89]. Information on estrogen receptor localisation in the spinal cord of males rats is sparse although it has been reported in the SPN before 4 weeks of age [90]. It has been suggested that androgens can exert an influence on motor neurones by acting as antagonists at glucocorticoid receptors. Immunoreactivity for glucocorticoid receptors has been demonstrated in the nuclei of SNB motor neurons in the male rat [91] and at levels more rostral to L5, in the cytoplasm and nuclei of motor neurons and associated glial cells in both sexes [92, 93]. There is one report of progesterone receptors in the cytoplasm of motor neurons in intact male rats [94] though this was not confirmed by a second study using different antibodies [95]. In ovariectomised female rats, estrogen treatment induces nuclear progesterone receptor immunoreactivity in the DGC and intermediolateral cell column at L3-S1, which represents the SPN at this level [95]. The distribution of androgen receptors in the lumbosacral spinal cord of males has been extensively discussed above. In females, androgen receptor expression is present in pudendal motor neurons that innervate the external urethral sphincter [96], however the micrographs in this paper suggest that the labelling is primarily cytoplasmic. The significance of this (if any) for continence control is unknown.
It seems likely that the greater number of SRC-1 expressing cells in male rats than in females, is attributable to the greater number of cells that express PG-21 and are involved in male sexual reflexes. The age-related decline in SRC-1 nuclear expressing cells in intact male rats matches the decline in PG-21 expression. It has previously been claimed that the number of SRC-1 expressing cells in the SNB falls by 40% in aged rats [66]. The much smaller decline seen here may reflect differences in criteria for identifying immunolabelled cells as Matsumoto [66] included very lightly stained cells in the sample he used for analysis. Interestingly, orchidectomy five days prior to perfusion, whether followed by TP replacement or not, did not cause significant changes in SRC-1 expression in any of the lumbosacral motor columns. This may indicate that these motor neurons are still responsive to circulating glucocorticoids (the animals were not adrenalectomised) and that SRC-1 remains involved in promoting the nuclear translocation of glucorticoid receptors which are also known to be present in pudendal motor neurons [91]. Alternatively SRC-1, despite being present in the majority of SNB motor neurons [69, 97] may not in fact be linked to androgen responsiveness. In SRC-1 null mutant mice, the dimensions of SNB motor neuron somata are similar to those of wild type animals [98]. If the same is true for rats, then the decline in SRC-1 expression seen in the aged males may be independent of the progressive reduction in testosterone levels though still indicative of an age-related down-regulation of cellular activity, perhaps linked to the effects of other steroids or cell signalling pathways such as those involving CREB. An association with CREB pathways via CBP might also account for the fact that in the cells of DH and DGC, orchidectomy appears to lead to a slight increase in SRC-1 expression. This might be related to the up-regulation of pCREB observed within cells in the same region (see below).
Immunohistochemistry for pCREB revealed that in both sexes it was almost ubiquitously expressed in the nuclei of cells in both the grey and white matter of L5-S1. Nuclear labelling was present in putative motor neurons within the DLN, RDLN, SNB, and VMN. These were surrounded by other pCREB immunoreactive cells that were likely to be either glial cells or interneurons. Other regions examined, including the DGC, DH, and SPN, also displayed abundant labelling for pCREB. These observations support a brief prior report by Matsumoto [72] who also noted an age-related decline of about 40% in the numbers of immunopositive SNB motor neurons in male rats. We observed a similar (47%) decline in the SNB, as well as a reduction of 36% and 24% for motor neurons in the DLN and RDLN respectively, and an 18% decline in cells in the DGC which probably represented glia or interneurons. By contrast, the only significant decline in female rats was one of 10%, in regions of DH associated with the control of micturition or sexual function. This would support the argument that a long-standing decline in testosterone levels is necessary to induce the age-related attrition in pCREB expression. Acute changes due to orchidectomy appeared to exert much less influence on pudendal motor neurons as regardless of age, this did not affect the numbers of pCREB nuclear expressing cells. This is perhaps not surprising since a variety of physiological stimuli in addition to circulating androgens can lead to phosphorylation of pCREB, including cellular responses to stress, the actions of neurotransmitters (including glutamate) and neurotrophins such as brain-derived neutrophic factor (BDNF) [99-102]. Significant in this regard may be the above mentioned age-related reduction in the glutamatergic input to SNB motor neuron dendrites [44]. A similar down-regulation of pCREB expression may be linked to an absence of BDNF. Through its interactions with the high affinity receptor TrkB, BDNF can activate a series of cell signalling pathways that lead to the phosphorylation of CREB and thus the cellular changes underlying neuronal plasticity and long-term potentiation [58, 59, 103]. Immunoreactivity for TrkB receptors has been demonstrated within SNB motor neurons [104] while in situ hybridisation studies have revealed mRNA encoding TrkB in motor neurons of the DLN, RDLN, and SNB [105]. As BDNF levels are reduced in the spinal cord of aged rats [61] and TrkB mRNA expression falls by approximately 25% in motor neurons of the DLN, RDLN, and SNB [105], this may account for some of the decrease in pCREB expression seen in the present study. This is probably exacerbated by the fact in pudendal motor neurons, BDNF and testosterone have an aggregative effect on soma size and dendritic morphology [106, 107]. Whether such processes play a role in senescence in other regions of the lumbosacral cord here remains to be explored.
Many factors influence pCREB expression and the increase in levels of expression following orchidectomy, which is most marked in the DH and DGC, could be a general effect of surgery as noxious stimulation of peripheral tissues has been shown to have this effect on spinal neurons, including presumptive nociceptive interneurons in the superficial laminae of the DH [108, 109]. The increase might also be related to the up-regulation of SRC-1 following orchidectomy. pCREB, through its interaction with CBP and hence with SRC-1, is able to influence the activity of steroid receptor responsive genes. By this means, it might facilitate SRC-1 expression.
Figures and Tables
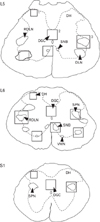 | Fig. 1Diagram showing the areas of the spinal cord (rectangles) analysed in the fifth and sixth lumbar (L5, L6) and first sacral (S1) segments. The dimensions of the rectangles are 120×120 µm (1), 180×180 µm (2), and 240×240 µm (3). DGC, dorsal grey commissure; DH, dorsal horn; DLN, dorsolateral nucleus; RDLN, retrodorsolateral nucleus; SNB, spinal nucleus of the bulbospongiosus; SPN, sacral parasympathetic nucleus, VMN, ventromedial nucleus. |
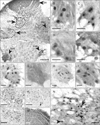 | Fig. 2PG-21 (androgen receptor) immunoreactivity within lumbosacral spinal cord. (A) Low power photomicrograph of PG-21 immunostaining in a section from a young male rat. Staining (black punctate dots) is seen predominantly within the nuclei of pudendal motor neurons of the dorsolateral nucleus (DLN) and spinal nucleus of the bulbospongiosus (SNB), and within the neurons of the dorsal grey commissure (DGC). Moderate numbers of cells in the dorsal horn (DH) and sacral parasympathetic nucleus (SPN) are immunoreactive. By contrast labelled motor neurons within the retrodorsolateral nucleus (RDLN) are sparser. Dashed lines indicate the approximate boundaries of the RDLN and SPN. (B-E) show labelled motor neurons in the SNB. More cells are labelled in intact young male rats (B) or those orchidectomised with testosterone replacement (C) than in aged intact males (D). Testosterone replacement is also effective in orchidectomised aged rats (E) although expression does not match that seen for young animals. (F-I) show motor neurons within the DLN. Young male rats show robust nuclear immunoreactivity for PG-21 which is completely abolished by orchidectomy (G) and only partially restored by testosterone propionate treatment (H). The number of PG-21 immunoreactive motor neurons within the DLN of aged male rats (I) is significantly smaller than in young rats (F). A similar situation prevails in the DGC where numbers of PG-21 immunoreactive cells were significantly higher in young animals (J) compared to orchidectomised animals +TP treatment (K) and aged animals (L). CC, spinal central canal. In young males (M), labelled nuclei in the DH appeared largely confined to laminae I-III. (N) Immunoreactivity of nuclei within the SPN of an aged male rat. The larger nuclei (large arrowheads) probably belong to preganglionic neurons whilst the smaller nuclei (small arrowheads) may belong to glial cells or interneurons. Scale bars=200 µm (A), 50 µm (B-N). |
 | Fig. 3Graphs showing the number of cells with nuclei immunoreactive for PG-21 in segments L5-S1. Data is presented for young and aged (3 and 24 months) intact male rats and for orchidectomised animals (orchid) with and without testosterone propionate replacement (+TP). For the sacral parasympathetic nucleus (SPN) a distinction was made between immunoreactive cells with a diameter of 20-25 µm which probably represent preganglionic neurons (SPNp), and those that were smaller and likely to be interneurons or glial cells (SPNi). DGC, dorsal grey commissure; DH, dorsal horn; DLN, dorsolateral nucleus; SNB, spinal nucleus of the bulbospongiosus. |
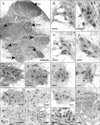 | Fig. 4Immunolocalisation of steroid receptor co-activator one (SRC-1) in lumbosacral spinal cord. (A) Section from L6 of a young intact male rat showing neurons with labelled nuclei distributed widely throughout the spinal grey matter. DH, dorsal horn. (B-E) Large labelled nuclei in the spinal nucleus of the bulbospongiosus (SNB) probably belong to motor neurons (e.g., black arrowhead in C) while smaller nuclei belong to glial cells or interneurons (white arrowhead). SRC-1 immunolabelling is robust in intact young male rats (B) and appears unaffected by orchidectomy (C), regardless of testosterone replacement (D), but the number of labelled nuclei is reduced by ageing (E). A similar relationship is seen in the dorsolateral nucleus (DLN) of young (F) vs. aged (G) intact male rats. There are no differences in numbers of labelled nuclei in DLN motor neurons between young (H) and aged (I) female rats. In (F-I), the larger cells (black arrowhead in G) are likely to be motor neurons whilst the smaller cells (white arrowhead in G) are probably glial cells or interneurons. The retrodorsolateral nucleus (RDLN) in male rats also appears susceptible to the effects of ageing since more motor neurons with labelled nuclei are present in young (J) than in aged animals (K). Black arrowheads indicate likely motor neurons whilst white arrowheads point to nuclei of interneurons or glia. A small group of L6 motor neurons with SRC-1-labelled are present in ventromedial nucleus (VMN) (L, young female rat). SRC-1 was abundant in laminae I-III of the dorsal horn (M, aged male rat). Many labelled nuclei were seen in the dorsal grey commissure (DGC) (N, aged male rat). The cell marked by the arrowhead is seen at higher magnification in the inset. (O) Labelling in the sacral parasympathetic nucleus (SPN). The highlighted area is shown at higher magnification in (P) in which putative preganglionic neurons (black arrowheads) can be distinguished from smaller cells (white arrowheads) that may represent interneurons or glial cells. (N-P) are from an aged male rat. Scale bars=200 µm (A), 50 µm (B-M), 100 µm (N, O), 25 µm (P). |
 | Fig. 5Graphs showing the numbers of steroid receptor co-activator one immunoreactive cells within lumbosacral spinal nuclei. Counts were performed in spinal sections derived from gonadally intact males and females aged 3 and 24 months and male rats that were orchidectomised (orchid) with or without testosterone replacement (+TP). Values are presented as mean±SEM. DGC, dorsal grey commissure; DH, dorsal horn; DLN, dorsolateral nucleus; i, interneurons orglial cells; p, preganglionic neurons; RDLN, retrodorsolateral nucleus; SNB, spinal nucleus of the bulbospongiosus; SPN, sacral parasympathetic nucleus; VMN, ventromedial nucleus. |
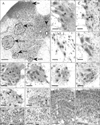 | Fig. 6Phosphorylated form of c-AMP response element binding protein (pCREB) localisation within lumbosacral spinal cord. Cells expressing immunoreactivity for pCREB are extensively located throughout the grey matter and are also observed to a lesser extent within the white matter (A, from a young female rat). VMN, ventral medial nucleus. (B-E) illustrate labelled cells within the region of the spinal nucleus of the bulbospongiosus (SNB) which are relatively few in females. (B) Probable motor neurons (black arrowhead) and interneurons or glial cells (white arrowhead) in an intact young male rat. Nuclear expression of pCREB appears similar to this in orchidectomised rats regardless of whether they did (D) or did not (C) receive testosterone injections. However the numbers of pCREB expressing motor neurons within the SNB appears reduced in aged (E) in comparison to young male rats (B). (F-I) show the immunolocalisation of pCREB within the dorsolateral nucleus. pCREB expressing motor neurons in young intact males (F) are present in greater numbers then in aged intact males (G) or either young (H) or aged (I) females, however there is no age-related decline in the females. (J) Nuclear immunoreactivity for pCREB in the retrodorsolateral nucleus (RDLN) of an aged male rat is seen both in putative motor neurons (black arrowhead) and other cells (glia or interneurons, white arrowhead). A similar situation prevails in the sacral parasympathetic nucleus (SPN) (K, young intact male). In the dorsal horn (DH), (L, M) cell nuclei are immunoreactive for pCREB are particularly abundant in laminae I-III (L, young intact male) and their numbers increase significantly following orchidectomy (M, young male). pCREB containing cells are also densely packed within the dorsal grey commissure (DGC) (N, O) and exhibit an age related decline in number (N, young intact male; O, aged intact male). Scale bars=200 µm (A), 50 µm (B-J), 100 µm (K-O). |
 | Fig. 7Graphs showing numbers of phosphorylated form of c-AMP response element binding protein immunoreactive cells in lumbosacral spinal nuclei. For each region data is provided for male and female rats, that are gonadally intact, and at two ages. Further data is provided for males that have been orchidectomised (orchid) with or without testosterone replacement (+TP). All counts represent mean±SEM. DGC, dorsal grey commissure; DH, dorsal horn; DLN, dorsolateral nucleus; i, interneurons orglial cells; p, preganglionic neurons; RDLN, retrodorsolateral nucleus; SNB, spinal nucleus of the bulbospongiosus; SPN, sacral parasympathetic nucleus; VMN, ventromedial nucleus. |
Acknowledgements
This work was funded by grant ERA16241 from the UK Biotechnology and Biological Sciences Research Council (BBSRC) awarded to RMS and AHDW.
References
1. Dering MA, Santer RM, Watson AH. Age-related changes in the morphology of preganglionic neurons projecting to the rat hypogastric ganglion. J Neurocytol. 1996. 25:555–563.
2. Hancock MB, Peveto CA. Preganglionic neurons in the sacral spinal cord of the rat: an HRP study. Neurosci Lett. 1979. 11:1–5.
3. Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984. 226:238–245.
4. Ranson RN, Santer RM, Watson AH. The relationship between serotonin, dopamine beta hydroxylase and GABA immunoreactive inputs and spinal preganglionic neurones projecting to the major pelvic ganglion of Wistar rats. Neuroscience. 2006. 141:1935–1949.
5. Santer RM, Dering MA, Ranson RN, Waboso HN, Watson AH. Differential susceptibility to ageing of rat preganglionic neurones projecting to the major pelvic ganglion and of their afferent inputs. Auton Neurosci. 2002. 96:73–81.
6. Yaici ED, Rampin O, Tang Y, Calas A, Jestin A, Leclerc P, Benoit G, Giuliano F. Catecholaminergic projections onto spinal neurons destined to the pelvis including the penis in rat. Int J Impot Res. 2002. 14:151–166.
7. Dering MA, Santer RM, Watson AH. Age-related changes in the morphology of preganglionic neurons projecting to the paracervical ganglion of nulliparous and multiparous rats. Brain Res. 1998. 780:245–252.
8. Papka RE, Newton BW, McNeill DL. Origin of galanin-immunoreactive nerve fibers in the rat paracervical autonomic ganglia and uterine cervix. J Auton Nerv Syst. 1991. 33:25–33.
9. Dail WG, Trujillo D, de la Rosa D, Walton G. Autonomic innervation of reproductive organs: analysis of the neurons whose axons project in the main penile nerve in the pelvic plexus of the rat. Anat Rec. 1989. 224:94–101.
10. Kepper M, Keast J. Immunohistochemical properties and spinal connections of pelvic autonomic neurons that innervate the rat prostate gland. Cell Tissue Res. 1995. 281:533–542.
11. Papka RE, Cotton JP, Traurig HH. Comparative distribution of neuropeptide tyrosine-, vasoactive intestinal polypeptide-, substance P-immunoreactive, acetylcholinesterase-positive and noradrenergic nerves in the reproductive tract of the female rat. Cell Tissue Res. 1985. 242:475–490.
12. Papka RE, McCurdy JR, Williams SJ, Mayer B, Marson L, Platt KB. Parasympathetic preganglionic neurons in the spinal cord involved in uterine innervation are cholinergic and nitric oxide-containing. Anat Rec. 1995. 241:554–562.
13. Papka RE, Traurig HH, Schemann M, Collins J, Copelin T, Wilson K. Cholinergic neurons of the pelvic autonomic ganglia and uterus of the female rat: distribution of axons and presence of muscarinic receptors. Cell Tissue Res. 1999. 296:293–305.
14. Purinton PT, Fletcher TF, Bradley WE. Gross and light microscopic features of the pelvic plexus in the rat. Anat Rec. 1973. 175:697–705.
15. Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997. 389:584–602.
16. Nadelhaft I, Miranda-Sousa AJ, Vera PL. Separate urinary bladder and prostate neurons in the central nervous system of the rat: simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. BMC Neurosci. 2002. 3:8.
17. Vera PL, Nadelhaft I. Anatomical evidence for two spinal 'afferent-interneuron-efferent' reflex pathways involved in micturition in the rat: a 'pelvic nerve' reflex pathway and a 'sacrolumbar intersegmental' reflex pathway. Brain Res. 2000. 883:107–118.
18. Marson L, Cai R, Makhanova N. Identification of spinal neurons involved in the urethrogenital reflex in the female rat. J Comp Neurol. 2003. 462:355–370.
19. Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am J Physiol Regul Integr Comp Physiol. 2006. 291:R419–R428.
20. Marson L, Carson CC 3rd. Central nervous system innervation of the penis, prostate, and perineal muscles: a transneuronal tracing study. Mol Urol. 1999. 3:43–50.
21. Marson L, Gravitt K. Spinal neurons activated with the urethrogenital reflex in the male rat. Brain Res. 2004. 1026:108–115.
22. Yaïci ED, Rampin O, Calas A, Jestin A, McKenna KE, Leclerc P, Benoit G, Giuliano F. alpha(2a) and alpha(2c) adrenoceptors on spinal neurons controlling penile erection. Neuroscience. 2002. 114:945–960.
23. Berkley KJ, Hubscher CH, Wall PD. Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J Neurophysiol. 1993. 69:545–556.
24. Blok BF. Central pathways controlling micturition and urinary continence. Urology. 2002. 59:5 Suppl 1. 13–17.
25. Giuliano F, Rampin O. Neural control of erection. Physiol Behav. 2004. 83:189–201.
26. Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982. 249:309–314.
27. McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986. 248:532–549.
28. Schrøder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980. 192:567–587.
29. Holmes GM, Rogers RC, Bresnahan JC, Beattie MS. External anal sphincter hyperreflexia following spinal transection in the rat. J Neurotrauma. 1998. 15:451–457.
30. Fraser MO, Chancellor MB. Neural control of the urethra and development of pharmacotherapy for stress urinary incontinence. BJU Int. 2003. 91:743–748.
31. Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985. 229:671–673.
32. Sengelaub DR, Arnold AP. Hormonal control of neuron number in sexually dimorphic spinal nuclei of the rat: I. Testosterone-regulated death in the dorsolateral nucleus. J Comp Neurol. 1989. 280:622–629.
33. Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980. 210:564–566.
34. Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981. 225:297–307.
35. Tobin AM, Payne AP. Perinatal androgen administration and the maintenance of sexually dimorphic and nondimorphic lumbosacral motor neuron groups in female Albino Swiss rats. J Anat. 1991. 177:47–53.
36. Leslie M, Forger NG, Breedlove SM. Sexual dimorphism and androgen effects on spinal motoneurons innervating the rat flexor digitorum brevis. Brain Res. 1991. 561:269–273.
37. Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983. 217:75–85.
38. Hodges LL, Jordan CL, Breedlove SM. Hormone-sensitive periods for the control of motoneuron number and soma size in the dorsolateral nucleus of the rat spinal cord. Brain Res. 1993. 602:187–190.
39. Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J Neurobiol. 1991. 22:976–988.
40. Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990. 10:935–946.
41. Goldstein LA, Sengelaub DR. Motoneuron morphology in the dorsolateral nucleus of the rat spinal cord: normal development and androgenic regulation. J Comp Neurol. 1993. 338:588–600.
42. Fargo KN, Iwema CL, Clark-Phelps MC, Sengelaub DR. Exogenous testosterone reverses age-related atrophy in a spinal neuromuscular system. Horm Behav. 2007. 51:20–30.
43. Matsumoto A. Synaptic changes in the perineal motoneurons of aged male rats. J Comp Neurol. 1998. 400:103–109.
44. Ranson RN, Santer RM, Watson AH. Ageing reduces the number of vesicular glutamate transporter 2 containing immunoreactive inputs to identified rat pelvic motoneurons. Exp Gerontol. 2007. 42:506–516.
45. Ghanadian R, Lewis JG, Chisholm GD. Serum testosterone and dihydrotestosterone changes with age in rat. Steroids. 1975. 25:753–762.
46. Matsumoto A, Prins GS. Age-dependent changes in androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Neurosci Lett. 1998. 243:29–32.
47. Matsumoto A, Prins GS. Androgenic regulation of expression of androgen receptor protein in the perineal motoneurons of aged male rats. J Comp Neurol. 2002. 443:383–387.
48. Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992. 26:110–135.
49. McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002. 108:465–474.
50. Shibata H, Spencer TE, Oñate SA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997. 52:141–164.
51. Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000. 245:1–11.
52. Smith CL, Oñate SA, Tsai MJ, O'Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci U S A. 1996. 93:8884–8888.
53. Wu RC, Smith CL, O'Malley BW. Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr Rev. 2005. 26:393–399.
54. McManus KJ, Hendzel MJ. CBP, a transcriptional coactivator and acetyltransferase. Biochem Cell Biol. 2001. 79:253–266.
55. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989. 59:675–680.
56. Yamamoto KK, Gonzalez GA, Biggs WH 3rd, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988. 334:494–498.
57. Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990. 13:184–188.
58. Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000. 25:11–14.
59. Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006. 361:1545–1564.
60. Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002. 82:637–672.
61. Ulfhake B, Bergman E, Edstrom E, Fundin BT, Johnson H, Kullberg S, Ming Y. Regulation of neurotrophin signaling in aging sensory and motoneurons: dissipation of target support? Mol Neurobiol. 2000. 21:109–135.
62. Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001. 4:981–988.
63. Jordan C. Androgen receptor (AR) immunoreactivity in rat pudendal motoneurons: implications for accessory proteins. Horm Behav. 1997. 32:1–10.
64. Jordan CL, Padgett B, Hershey J, Prins G, Arnold A. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J Comp Neurol. 1997. 379:88–98.
65. Lubischer JL, Arnold AP. Axotomy transiently down-regulates androgen receptors in motoneurons of the spinal nucleus of the bulbocavernosus. Brain Res. 1995. 694:61–68.
66. Matsumoto A. Age-related changes in nuclear receptor coactivator immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Brain Res. 2002. 943:202–205.
67. Matsumoto A, Arai Y, Prins GS. Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J Neuroendocrinol. 1996. 8:553–559.
68. Watkins TW, Keast JR. Androgen-sensitive preganglionic neurons innervate the male rat pelvic ganglion. Neuroscience. 1999. 93:1147–1157.
69. O'Bryant EL, Jordan CL. Expression of nuclear receptor coactivators in androgen-responsive and -unresponsive motoneurons. Horm Behav. 2005. 47:29–38.
70. Ranson RN, Priestley DJ, Santer RM, Watson AH. Changes in the substance P-containing innervation of the lumbosacral spinal cord in male Wistar rats as a consequence of ageing. Brain Res. 2005. 1036:139–144.
71. Ranson RN, Santer RM, Watson AH. SRC-1 localisation in lumbosacral spinal cord of male and female Wistar rats. Neuroreport. 2003. 14:1821–1824.
72. Matsumoto A. Age-dependent changes in phosphorylated cAMP response element-binding protein immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Neurosci Lett. 2000. 279:117–120.
73. Gréco B, Edwards DA, Zumpe D, Michael RP, Clancy AN. Fos induced by mating or noncontact sociosexual interaction is colocalized with androgen receptors in neurons within the forebrain, midbrain, and lumbosacral spinal cord of male rats. Horm Behav. 1998. 33:125–138.
74. Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995. 359:443–456.
75. Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999. 414:167–192.
76. Eika B, Levin RM, Longhurst PA. Modulation of urinary bladder function by sex hormones in streptozotocin-diabetic rats. J Urol. 1994. 152(2 Pt 1):537–543.
77. Collins WF 3rd, Seymour AW, Klugewicz SW. Differential effect of castration on the somal size of pudendal motoneurons in the adult male rat. Brain Res. 1992. 577:326–330.
78. Chambers KC, Phoenix CH. Testosterone and the decline of sexual behavior in aging male rats. Behav Neural Biol. 1984. 40:87–97.
79. Chambers KC, Phoenix CH. Testosterone is more effective than dihydrotestosterone plus estradiol in activating sexual behavior in old male rats. Neurobiol Aging. 1986. 7:127–132.
80. Davidson JM, Stefanick ML, Sachs BD, Smith ER. Role of androgen in sexual reflexes of the male rat. Physiol Behav. 1978. 21:141–146.
81. Frankel AI, Mock EJ. Time course of hormonal response to sexual behavior in aging male rats. Exp Gerontol. 1981. 16:363–369.
82. Gray GD. Age-related changes in penile erections and circulating testosterone in middle-aged male rats. Adv Exp Med Biol. 1978. 113:149–158.
83. Meisel RL, O'Hanlon JK, Sachs BD. Differential maintenance of penile responses and copulatory behavior by gonadal hormones in castrated male rats. Horm Behav. 1984. 18:56–64.
84. Hsu HK, Hsu C, Yu JY, Peng MT. Effects of long-term testosterone replacement on copulatory activity in old male rats. Gerontology. 1986. 32:10–17.
85. Ranson RN, Dodds AL, Smith MJ, Santer RM, Watson AH. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003. 972:149–158.
86. Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci Lett. 1995. 196:25–28.
87. Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001. 304:193–214.
88. VanderHorst VG, Meijer E, Holstege G. Estrogen receptor-alpha immunoreactivity in parasympathetic preganglionic neurons innervating the bladder in the adult ovariectomized cat. Neurosci Lett. 2001. 298:147–150.
89. Williams SJ, Papka RE. Estrogen receptor-immunoreactive neurons are present in the female rat lumbosacral spinal cord. J Neurosci Res. 1996. 46:492–501.
90. Burke KA, Schroeder DM, Abel RA, Richardson SC, Bigsby RM, Nephew KP. Immunohistochemical detection of estrogen receptor alpha in male rat spinal cord during development. J Neurosci Res. 2000. 61:329–337.
91. Blanco CE, Peltz A, Staley R, Kim F. Effects of pharmacologic androgen treatment duration on glucocorticoid receptor alpha immunoreactivity of lumbosacral motor neurons in the male rat. Neuroscience. 2002. 115:941–949.
92. Ferrini M, Gonzalez S, Antakly T, De Nicola AF. Immunocytochemical localization of glucocorticoid receptors in the spinal cord: effects of adrenalectomy, glucocorticoid treatment, and spinal cord transection. Cell Mol Neurobiol. 1993. 13:387–397.
93. Yan P, Xu J, Li Q, Chen S, Kim GM, Hsu CY, Xu XM. Glucocorticoid receptor expression in the spinal cord after traumatic injury in adult rats. J Neurosci. 1999. 19:9355–9363.
94. Labombarda F, Guennoun R, Gonzalez S, Roig P, Lima A, Schumacher M, De Nicola AF. Immunocytochemical evidence for a progesterone receptor in neurons and glial cells of the rat spinal cord. Neurosci Lett. 2000. 288:29–32.
95. Monks DA, Arciszewska G, Watson NV. Estrogen-inducible progesterone receptors in the rat lumbar spinal cord: regulation by ovarian steroids and fluctuation across the estrous cycle. Horm Behav. 2001. 40:490–496.
96. Blanchet P, Yaici el D, Cayzergues L, Giuliano F, Jardin A, Benoit G, Droupy S. Identification of androgen receptors in the motoneurons of the external urethral sphincter in the spinal cord of female rats. Eur Urol. 2005. 47:118–124.
97. Ranson RN, Santer RM, Watson AH. Biogenic amine and neuropeptide inputs to identified pelvic floor motoneurons that also express SRC-1. Neurosci Lett. 2005. 382:248–253.
98. Monks DA, Xu J, O'Malley BW, Jordan CL. Steroid receptor coactivator-1 is not required for androgen-mediated sexual differentiation of spinal motoneurons. Neuroendocrinology. 2003. 78:45–51.
99. Balazs R. Trophic effect of glutamate. Curr Top Med Chem. 2006. 6:961–968.
100. De Cesare D, Fimia GM, Sassone-Corsi P. Signaling routes to CREM and CREB: plasticity in transcriptional activation. Trends Biochem Sci. 1999. 24:281–285.
101. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001. 2:599–609.
102. Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996. 15:4629–4642.
103. Ernfors P, Bramham CR. The coupling of a trkB tyrosine residue to LTP. Trends Neurosci. 2003. 26:171–173.
104. Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007. 85:303–309.
105. Johnson H, Hokfelt T, Ulfhake B. Decreased expression of TrkB and TrkC mRNAs in spinal motoneurons of aged rats. Eur J Neurosci. 1996. 8:494–499.
106. Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000. 44:308–319.
107. Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004. 145:161–168.
108. Anderson LE, Seybold VS. Phosphorylated cAMP response element binding protein increases in neurokinin-1 receptor-immunoreactive neurons in rat spinal cord in response to formalin-induced nociception. Neurosci Lett. 2000. 283:29–32.
109. Wu J, Su G, Ma L, Zhang X, Lei Y, Li J, Lin Q, Fang L. Protein kinases mediate increment of the phosphorylation of cyclic AMP-responsive element binding protein in spinal cord of rats following capsaicin injection. Mol Pain. 2005. 1:26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download