Abstract
Fetal development of the cartilage of the pharyngotympanic tube (PTT) is characterized by its late start. We examined semiserial histological sections of 20 human fetuses at 14-18 weeks of gestation. As controls, we also observed sections of 5 large fetuses at around 30 weeks. At and around 14 weeks, the tubal cartilage first appeared in the posterior side of the pharyngeal opening of the PTT. The levator veli palatini muscle used a mucosal fold containing the initial cartilage for its downward path to the palate. Moreover, the cartilage is a limited hard attachment for the muscle. Therefore, the PTT and its cartilage seemed to play a critical role in early development of levator veli muscle. In contrast, the cartilage developed so that it extended laterally, along a fascia-like structure that connected with the tensor tympani muscle. This muscle appeared to exert mechanical stress on the initial cartilage. The internal carotid artery was exposed to a loose tissue facing the tubal cartilage. In large fetuses, this loose tissue was occupied by an inferior extension of the temporal bone to cover the artery. This later-developing anterior wall of the carotid canal provided the final bony origin of the levator veli palatini muscle. The tubal cartilage seemed to determine the anterior and inferior margins of the canal. Consequently, the tubal cartilage development seemed to be accelerated by a surrounding muscle, and conversely, the cartilage was likely to determine the other muscular and bony structures.
In adults, the cartilage of the pharyngotympanic tube (PTT) or simply, the tubal cartilage, covers the superior and posterior aspects of the PTT: it resembles a cap, protecting the tube against mechanical stress. However, in contrast to the tracheal and laryngeal cartilages, the tubal cartilage may bend too acutely to maintain a wide luminal space. Thus, our first motivation of this study was to consider why the tubal cartilage is not specified for the "aim." In our recent studies of fetal anatomy, including the PTT [1, 2], we have found that development of the tubal cartilage starts much later than that of the other head and neck cartilages. Although it is perhaps not widely known, the tubal cartilage begins to develop at 14-16 weeks of gestation [3], whereas the PTT develops very early. Therefore, we hypothesized that, in contrast to most of the head and neck, whose development is determined by gene cascades for regulation of the pharyngeal arch [4-7], development of the tubal cartilage is strongly dependent on later developmental events occurring along or around the PTT. Therefore, in the present study using human fetuses, we considered the converse situation of whether any problem would arise along or around the fetal PTT, if the tubal cartilage was absent.
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined semiserial histological sections of 20 human fetuses at 14-18 weeks (crown-rump length [CRL], 100-150 mm) and 5 fetuses at around 30 weeks (CRL, 250-270 mm). Among the former specimens, the heads had been processed for horizontal (10 fetuses), frontal (5 fetuses) or sagittal (5 fetuses) sections. The latter 5 specimens (all sagittal sections) were used to confirm the final morphology at and around the PTT, because the morphology of the temporal bone at 14-18 weeks is quite different from that at the final stage [8]. All of the specimens were part of a large collection kept at Embryology Institute of the Universidad Complutense, Madrid, being the products of urgent abortion, miscarriages or ectopic pregnancies managed at the Department of Obstetrics of the University. Approval for the study was granted by the ethics committee of the university.
The donated fetuses were fixed with 10% v/v formalin solution for more than 3 months. After division into the head and neck, thorax, abdomen and pelvis, and four extremities, the head specimens were decalcified by incubating them at room temperature using Plank-Rychlo solution (AlCl2/6H2O, 7.0 w/v%; HCl, 3.6; HCOOH, 4.6) for 3-5 days [9]. After routine procedures for paraffin-embedded histology, sections 5 or 10 micron meter thick were prepared at intervals of 0.1-1 mm, depending on the size of the specimen. The sections included not only the PTT, but also any surrounding structures, such as parts of the brain, nose, ear and eye. All sections were stained with hematoxylin and eosin or azan.
Figs. 1, 2, 3 show horizontal sections, Fig. 4 frontal sections and Fig. 5 sagittal sections. The initial appearance of the tubal cartilage is evident in Fig. 1, and a fascial connection between the cartilage and the tensor tympani muscle is evident in Figs. 1 and 2. Likewise, the topographical relationship between the cartilage and the levator veli palatini muscle is shown in Figs. 1, 3, and 4. Finally, Fig. 5 displays the pharyngeal recess. In addition, all the figures show the underdeveloped carotid canal of the temporal bone, in contrast to the late stage (Fig. 5D inset).
In 1 of 20 specimens at 14-18 weeks, the tubal cartilage was almost round and 1 mm in diameter, being restricted to the posterior side of the pharyngeal opening of the PTT (102 mm CRL, at 14 weeks) (Fig. 1). We examined another 4 fetuses that were smaller than 110 mm (i.e., at approximately 14 weeks): The three of four specimens carried a short bar-like cartilage, extending laterally from the pharyngeal opening of the PTT (e.g., Fig. 5A, B), but the remaining specimen carried no cartilage along the PTT (Fig. 3A). Thus, it was considered that initially, the cartilage probably appeared as the medial end of the final long bar at or around 14 weeks, or at the CRL 100-110 mm stage, and then extended laterally. The initial cartilage was located at the medial end of a band-like mesenchymal condensation, which extended laterally to the tensor tympani muscle (see below).
The initial round cartilage developed into a bar-like cartilage extending laterally along a fascia-like structure (Fig. 2), which first appeared as a thick band-like mesenchymal condensation (Fig. 1B, C, F, G); this early morphology was similar to a type of PTT "adventitia." However, until 18 weeks, it was visible as a fascia joining the covering fascia of the tensor tympani muscle. The cartilage-tensor tympani fascia was located on the posterosuperior side of the PTT, and the composite fibers appeared to run along the mediolateral axis. In contrast, the anterior part of the PTT adventitia connected between the tensor veli palatini muscle and Meckel's cartilage (Fig. 1C, D). Notably, during the stages examined (14-18 weeks), the tensor tympani muscle had no bony attachment because, along and near the PTT, the putative temporal bone was not developed and was restricted to the area around the cochlea (which is a part derived from the otic capsule). Thus, the medial end of the tensor tympani muscle faced, or was attached to, the lateral end of the developing PTT (Fig. 2).
The site of the initial cartilage corresponded to the immediately superior side of the developing levator veli palatini muscle (Fig. 1D, H). Before the PTT cartilage appeared, the levator muscle entered a narrow mucosal fold protruding sharply at the posterior angle of the pharyngeal opening of the PTT (Fig. 3A). Later, depending on the development of the cartilage, the mucosal fold became thick with glands (Fig. 3B, C). Notably, the levator muscle used the mucosal fold for downward passage to the palate: this was another reason for the fold becoming thick. However, the salpingopalatine fold was not evident in the fetuses examined. Notably, instead of the bony attachment, the superior end (the putative origin from the temporal bone) of the levator muscle originated from a thick fascia, whose fiber components ran in the mediolateral direction along the inferior aspect of the PTT (Fig. 4). This inferior fascia was separated from the other peritubal fascia, which was connected with the tensor tympani muscle, but in both, the composite fibers ran along the mediolateral axis (see the paragraph above). Moreover, part of the levator muscle was attached to the cartilage itself (Fig. 4B, C). On the medial side, the levator muscle issued a fascia that reached the anterior aspect of the longus capitis muscle (Fig. 3C). In addition, the tensor veli palatini muscle issued a fascia that extended laterally to Meckel's cartilage (Figs. 1C, 2C), as well as medially to the pterygoid process of the sphenoid bone (Fig. 3A, B). However, the fascia of the tensor veli muscle did not connect with the tubal cartilage at the early stage. Likewise, the origin of the tensor veli muscle from the lateral lamina of the tubal cartilage was not evident in the early stage of development. The tensor veli palatini muscle originated from the pterygoid process, which had already started to ossify at 14 weeks.
At 14-18 weeks, the internal carotid artery ran superiorly along the anterior surface of the cartilaginous cochlea. Thus, the artery was exposed to loose mesenchymal tissue, facing the PTT and tubal cartilage (Figs. 1, 2, 3, 5B, C); this tissue mass, or a loose space, was located on the posterosuperior side of the PTT (Figs. 2-5). Notably, in the large fetuses examined, the space was completely occupied by the later-developing part of the temporal bone; thus, the bony carotid canal was established (Fig. 5D inset), and the loose space was replaced by the anterior wall of the bony carotid canal. Subsequently, the levator veli palatini muscle acquired its bony attachment to the inferior extension of the temporal bone.
The pharyngeal recess, or Rosenműller's fossa, of the pharyngeal cavity was clearly identifiable in sagittal sections (Fig. 5A, C). The PTT cartilage was sandwiched between the recess and the PTT. In horizontal sections, the recess corresponded to part of the pharyngeal cavity behind a large mucosal fold containing the cartilage and levator muscle (Fig. 3C). The shape and size of the pharyngeal recess did not show individual variation in the fetuses examined, in contrast to the observations of adults by Khoo et al. [10]. The salpingopharyngeal fold was not evident in fetuses, but the salpingopharyngeal muscle was identified as a medial part of the constrictor pharyngis superior muscle (Fig. 4).
The present study demonstrated that the tubal cartilage developed along a peritubal fascia connecting with the tensor tympani muscle. Likewise, the levator veli palatini muscle originated from another peritubal fascia on the inferior side of the PTT. Because their composite fibers were oriented in the same mediolateral direction, these peritubal fasciae were likely to be mutually continuous in the posterior aspect. In addition, a similar peritubal fascia was evident between the tensor veli palatini muscle and Meckel's cartilage. Thus, instead of the later-developing bony attachments, the PTT provided fascial structures for all of the three paratubal muscles. The peritubal fasciae seemed to develop as a migration fascia against a growing structure (i.e., the PTT), being apparently analogous to the renal fascia against the developing kidney [11, 12]. However, unlike the renal fascia, the paratubal muscles used the fasciae as an origin at the early stage. The fascia for the levator veli palatini muscle origin extended medially to the prevertebral fascia covering the longus capitis muscle. Miyake et al. [13] reported that the latter develops as a kind of intermediate tendon between the bilateral prevertebral muscles. Thus, multiple fasciae with different origins might be interconnected.
In adults, it is well known that the tensor veli palatini, as well as the tensor tympani, becomes attached to the tubal cartilage [14]. However, the tensor veli muscle origin from the lateral lamina of the tubal cartilage was not evident during early development. It has been noted that the levator veli in both the adult and the newborn originates via a cylindrical tendon attached to the inferior surface of the petrous part of the temporal bone near the carotid foramen, and also to the tubal cartilage [15]. However, instead of the petrosal part, a loose space was noted between the internal carotid artery and the PTT at 14-18 weeks. Klueber and Langdon [16] reported that, in 15-week human fetuses, the levator veli muscle sometimes originates from the membranous tube, although they did not describe the peritubal fasciae. We observed the tubal cartilage as a limited hard tissue that was available for the origins of the developing levator veli and tensor tympani muscles. Conversely, via the peritubal fasciae, the tubal cartilage at the early stage seemed to receive mechanical stress for growth. The tensor tympani muscle appeared likely to lead or induce lateral extension of the cartilage along the fasciae, whereas the levator veli muscle was likely to provide cues for extension of the medial lamina of the cartilage. Therefore, the tubal cartilage seems to start development after the establishment of paratubal muscles. The PTT developed first, paratubal muscles second, and the tubal cartilage third.
Swarts et al. [3] reported that the distances of the levator veli and tensor veli muscles from the tubal lumen remain essentially unchanged throughout development, when standardized relative to the changes in body size. Moreover, the topographical anatomy of the PTT and paratubal muscle origins in normal newborns and those with cleft palate is almost the same [17]. Such stable morphology of the paratubal muscles seemed to be a result of the early development, in accordance with the PTT. Subsequently, the tubal cartilage morphology is also stable, although after birth, the shape begins to show a difference between the normal situation and that of the cleft palate [18]. Mallo et al. [19] demonstrated a critical role of the epithelium in the external ear cartilage pattern formation. In fact, the initial cartilage bars develop along the external auditory meatus, and surround it (now submitted to Ann Anat). However, development of the tubal cartilage might not depend on the differentiation of the PTT epithelium, including the glands. Rather than having a positive influence, the glands often invaded into the tubal cartilage, and sometimes divided the latter, as has been reported in the epiglottic cartilage [2]. The invasion of glands may be related to the distribution of elastic fibers, those developed in the late stages in the fetal tubal cartilage (unpublished data).
On the basis of our findings and the available data, we hypothesize that 1) one, the PTT and paratubal muscles differentiate through predetermined gene cascades that are responsible for development of the first and second pharyngeal arches; 2) the other, the tubal cartilage mechanically supports the development of paratubal muscle, and vice versa.
Figures and Tables
Fig. 1
Initial stage of development of the pharyngotympanic tube cartilage: a fetus with 102 mm crown-rump length. Hematoxlyin and eosin staining. Horizontal sections. (A [E]) is the most superior (or inferior) side of the figure: the distance is 3 mm. The posterior or lateral orientation is shown by arrows in (D). (F-H) are higher-magnigfication views of the pharyngotympanic tube (PTT) shown in (B-D), respectively. (A [C]) displays the superior (or inferior) end of the tubal cartilage (C): thus, the initial cartilage, almost 1 mm in diameter, is restricted to the posterior side of the pharyngeal opening of the PTT. The superior end of the levator veli palatini muscle (LVP) is seen 1 mm below the cartilage (D). A thick, band-like mesenchymal condensation or an "adventitia" in the text (triangles) extends between the cartilage and the tensor tympani muscle (TT). A facia (arrows) connects between the tensor veli palatini muscle (TVP) and Meckel's cartilage (MC). The internal cartotid artery (ICA) does not run through the bony carotid canal but is exposed to a large loose space (stars) on the posterior side of the PTT. ATN, auriculotemporal nerve; LP, lateral pterygoid muscle; MN, mandibular nerver; MP, medial pterygoid muscle; OG, otic ganglion; PT, pterygoid process; TMJ, temporomandibular joint. Scale bars in (A)=1 mm (A-E); in (F)=0.5 mm (F-H).

Fig. 2
(A-C) Laterally extending cartilage and the tensor tympani muscle in a crown-rump length 150-mm fetus. Hematoxylin and eosin staining. Horizontal sections. (A [C]) is the most inferior (or superior) side of the figure: the distance is 2 mm. The posterior or lateral orientation is shown by the arrows in (C). The cartilage (C) of the pharyngotympanic tube (PTT) extends along a fascial structure or an adventitia (triangles) that connects with the tensor tympani muscle (TT). Arrows indicate another fascia connecting between the tensor veli palatini muscle (TVP) and Meckel's cartilage (MC). The internal cartotid artery (ICA) is exposed to a loose space (stars) on the posterior side of the PTT. ATN, auriculotemporal nerve; LC, longus capitis muscle; LP, lateral pterygoid muscle; MP, medial pterygoid muscle; PT, pterygoid process. Scale bar=1 mm (A-C).
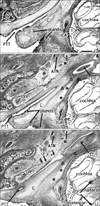
Fig. 3
Mucosal fold of the lateral pharyngeal wall and pharyngotympanic tube cartilage: three fetuses. Azan staining. Horizontal sections. All panels exhibit the inferior part or wall of the pharyngotympanic tube (PTT). The cartilage has not yet appeared in the specimen shown in (A) (crown-rump length [CRL], 101 mm), but the levator veli palatini muscle (LVP) has begun to extend into the putative mucosal fold at the pharyngeal opening of the PTT. In (B) (CRL, 100 mm) and (C) (CRL, 116 mm), the tubal cartilage (C) is almost 2 mm in length and these panels show the medial end. The LVP employs the mucosal fold containing the cartilage to guide its downward path to the palate. A fascia (circles), along the LVP, extends posteromedially to cover the longus capitis muscle (LC, panel C). Arrows indicate another fascia along the tensor veli palatini muscle (TVP). The internal cartotid artery (ICA) is exposed to a loose space (stars) on the posterior side of the PTT. LP, lateral pterygoid muscle; MP, medial pterygoid muscle; PT, pterygoid process;TB, temporal bone. Scale bar=1 mm (A-C).
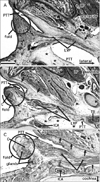
Fig. 4
Pharyngotympanic tube and the levator veli palatini muscle in a crown-rump length 125-mm fetus. Hematoxylin and eosin staining. Frontal sections. (A [E]) is the most posterior (or anterior) side of the figure: the distance is 3.3 mm. The superior or lateral orientation is shown by arrows in (A). The internal carotid artery (ICA with arrow) enters the brain cavity immediately posterior to (A). (A) and (B) display the most superior part or the origin (arrowheads) of the levator veli palatini muscle (LVP): because of the lack of a bony structure (star) near the pharyngotympanic tube (PTT), the origin has not yet become attached to the temporal bone, but extends along the inferior aspect of the PTT. The tubal cartilage (C) is divided into two fragments in (B). The levator muscle extends anteriorly along the inferior aspect of the PTT and its cartilage (C-E). ATN, auriculotemporal nerve; CPS, constrictor pharyngis superior muscle; ECA, external carotid artery; ICN, internal carotid nerve; MC, Meckel's cartilage; MH, mylohyoideus muscle; MP, medial pterygoid muscle; OG, otic ganglion; PG, parotid gland; SB, sphenoid bone (body); SG, styloglossus muscle; SMG, submandibular gland; SP, salpingopharyngeus muscle; TG, trigeminal nerve ganglion; TT, tensor tympani muscle; TVP, tensor veli palatini muscle; VN, Vidian's nerve (nerve of the pterygoid canal). Scale bar=1 mm (A-E).
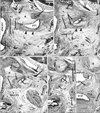
Fig. 5
Pharyngeal recess and the pharyngotympanic tube cartilage: two fetuses. Azan staining. Sagittal sections. (A and B [or C and D]) display fetuses with a crown-rump length of 103 mm (or 100 mm). (A) and (C) are located medially to (B) and (D), respectively. The superior or posterior orientation is shown by arrows in (A). The pharyngotympanic tube (PTT) and the tubal cartilage (C) are located between the pharyngeal recess (PR) and the PTT (A, B). Instead of the bony carotid canal, a loose space (stars in B and D) is evident between the PTT cartilage and the cochlea. Thus, the internal carotid artery (ICA) is exposed to the loose space. An inset (lower angle of D) displays a control specimen (30 wk) in which the bony carotid canal is established: the levator veli palatini muscle (LVP) attaches to the bony part that develops later (asterisks). CPM, constrictor pharyngis medius muscle; CPS, constrictor pharyngis superior muscle; FA, facial artery; HB, hyoid bone; LC, longus capitis muscle; LP, lateral pterygoid muscle; MC, Meckel's cartilage; MP, medial pterygoid muscle; PP, palatopharyngeal muscle; PT, pterygoid process; SG, styloglossus muscle; SH, stylohyoid muscle; SMG, submandibular gland; TVP, tensor veli palatini muscle. Scale bars=1 mm (A-D, inset in D).
Acknowledgements
This study is supported by a grant-in-aid from the Ministry of Science and Technology of Japan (No. 24592538 to Yukio Katori).
References
1. Katori Y, Rodríguez-Vázquez JF, Kawase T, Murakami G, Cho BH, Abe S. Early fetal development of hard tissue pulleys for the human superior oblique and tensor veli palatini muscles. Ann Anat. 2011. 193:127–133.
2. Katori Y, Takeuchi H, Rodríguez-Vázquez JF, Kitano H, Murakami G, Kawase T. Fetal development of the human epiglottis revisited: appearance of GFAP-positive mesenchymal cells and fibrous connections with other laryngeal and lingual structures. Ann Anat. 2011. 193:149–155.
3. Swarts JD, Rood SR, Doyle WJ. Fetal development of the auditory tube and paratubal musculature. Cleft Palate J. 1986. 23:289–311.
4. Couly G, Grapin-Botton A, Coltey P, Ruhin B, Le Douarin NM. Determination of the identity of the derivatives of the cephalic neural crest: incompatibility between Hox gene expression and lower jaw development. Development. 1998. 125:3445–3459.
5. Ruhin B, Creuzet S, Vincent C, Benouaiche L, Le Douarin NM, Couly G. Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm. Dev Dyn. 2003. 228:239–246.
6. Santagati F, Minoux M, Ren SY, Rijli FM. Temporal requirement of Hoxa2 in cranial neural crest skeletal morphogenesis. Development. 2005. 132:4927–4936.
7. Minoux M, Antonarakis GS, Kmita M, Duboule D, Rijli FM. Rostral and caudal pharyngeal arches share a common neural crest ground pattern. Development. 2009. 136:637–645.
8. Rodríguez-Vázquez JF, Murakami G, Verdugo-López S, Abe S, Fujimiya M. Closure of the middle ear with special reference to the development of the tegmen tympani of the temporal bone. J Anat. 2011. 218:690–698.
9. Plank J, Rychlo A. A method for quick decalcification. Zentralbl Allg Pathol. 1952. 89:252–254.
10. Khoo FY, Kanagasuntheram R, Chia KB. Variations of the lateral recesses of the nasopharynx. Arch Otolaryngol. 1967. 86:456–462.
11. Hayes MA. Abdominopelvic fasciae. Am J Anat. 1950. 87:119–161.
12. Matsubara A, Murakami G, Niikura H, Kinugasa Y, Fujimiya M, Usui T. Development of the human retroperitoneal fasciae. Cells Tissues Organs. 2009. 190:286–296.
13. Miyake N, Hayashi S, Kawase T, Cho BH, Murakami G, Fujimiya M, Kitano H. Fetal anatomy of the human carotid sheath and structures in and around it. Anat Rec (Hoboken). 2010. 293:438–445.
14. Abe M, Murakami G, Noguchi M, Kitamura S, Shimada K, Kohama GI. Variations in the tensor veli palatini muscle with special reference to its origin and insertion. Cleft Palate Craniofac J. 2004. 41:474–484.
15. Rohan RF, Turner L. The levator palati muscle. J Anat. 1956. 90:153–154.
16. Klueber K, Langdon HL. Anatomy of musculus levator veli palatini in the 15-week human fetus. Acta Anat (Basel). 1979. 105:94–105.
17. Doyle WJ, Kitajiri M, Sando I. The anatomy of the auditory tube and paratubal musculature in a one month old cleft palate infant. Cleft Palate J. 1983. 20:218–226.
18. Takasaki K, Sando I, Balaban CD, Ishijima K. Postnatal development of eustachian tube cartilage. A study of normal and cleft palate cases. Int J Pediatr Otorhinolaryngol. 2000. 52:31–36.
19. Mallo M, Schrewe H, Martin JF, Olson EN, Ohnemus S. Assembling a functional tympanic membrane: signals from the external acoustic meatus coordinate development of the malleal manubrium. Development. 2000. 127:4127–4136.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download