Abstract
Tonicity-responsive enhancer binding protein (TonEBP) is a signal transcription factor of transporters such as sodium-myo-inositol cotransporter (SMIT), aldose reductase. TonEBP has a variety of functions such as control of intracellular osmolytes and immunomodulating. It is known that TonEBP is abundant in the placenta, but location and function aren't known. The aim of this study is to describe the localization of TonEBP in the placenta. We assayed the immunohistochemistry of TonEBP and performed in situ hybridization of SMIT in normal human full term placenta. In normal human full term placenta, TonEBP was in villous trophoblasts, extravillous trophoblasts and some endothelial cells. The result of the in situ hybridization of SMIT was similar to that of immunohistochemistry of TonEBP. Neither TonEBP nor SMIT was present in TonEBP knockout mouse placenta. This shows TonEBP is a key factor in SMIT transcription. TonEBP may play an important role in transporting of inositol to fetus in placenta.
Tonicity-responsive enhancer binding protein/nuclear factor of activated T cell 5 (TonEBP/NFAT5) is a member of the Rel/NFkB/NFAT family of signal transcription factors [1]. It regulates transcription of transporters such as sodium-myo-inositol cotransporter (SMIT) and aldose reductase [2]. These transporters accumulate organic osmolytes (myo-inositol [Ins], sorbitol) into cells and help to increase intracellular osmolarity so cells can survive in hyperosmolar condition [3]. Under hypertonic condition, TonEBP moves into a nucleus and transcript those transporters to increase intracellular osmolarity [4]. The function of TonEBP is well-known in the kidney, but the other varied functions of TonEBP are under investigation, for example the immunomodulating function in T cell [5]. TonEBP is expressed in a wide variety of cell and tissue types, but most abundantly in the placenta [6].
Ins is important in membrane signaling and plays a special role in brain metabolism [7-10]. Ins transport from mother to fetus during fetal development is critical for brain development. In SMIT knockout mice, neonates die immediately after delivery due to central apnea [11]. Central apnea is associated with defective brainstem control of breathing: the pre-Botzinger respiratory control center discharges an abnormal electrical rhythm [11]. But SMIT knockout mice are rescued by feeding 1% inositol solution during pregnancy [12]. Berry et al. [13] found that SMIT is abundant in the placenta. Since Ins transportation mother to fetus passes through the placenta, abundant TonEBP mRNA and SMIT mRNA in placenta may play an important role in transporting Ins. The localization and function of TonEBP and SMIT is unknown in the placenta. The aim of this study is to localize TonEBP and SMIT in the human placenta and suggestfunction.
Human placentas were collected from women in full-term pregnancies who had been admitted for delivery at Ewha Womans University Hospital in Seoul, Korea, immediately after normal spontaneous vaginal delivery. The exclusion criteria in the study were: hypertension, diabetes, any complication of pregnancy in the pregnant women, premature and postterm babies, twins, and any developmental disease in the baby. Tissue sampling of placentas was obtained from selected 5 cases of normal term pregnancies. This study was approved by the Institutional Review Board of Ewha Womans University. Informed consent was obtained from all subjects.
A part of placenta was randomly selected and immediately fixed in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) for 12 hours, routinely processed and embedded in paraffin. Paraffin sections of 3 µm thickness were cut and deparaffinized in xylene and rehydrated with graded ethanol and water.
For collecting TonEBP knockout mouse placenta, 2 pregnant animals from heterozygote crossing delivered embryos by cesarean section, isolated placentas and took a piece of tail for genotyping at fetus 18.5 days. We collected 4 placentas from homozygotes, 3 placentas from wild types, 5 placentas from heterozygotes. The mouse placentas were processed in the same way as the human placenta.
An immunohistochemical study was performed to investigate localization of TonEBP expression.
TonEBP expression was detected by using the rabbit polyclonal antibodyagainst TonEBP. The antibody was raised in rabbits by using a commercial service (Covance, PA, USA) [1]. A mouse monoclonal antibody against cytokeratin 7 (Dako, Carpinteria, CA, USA) and a mouse monoclonal antibody against vimentin (Novocastra, Newcastle, UK) were used as a marker for the trophoblasts and the decidual cell, respectively [14]. Negative controls were performed by replacing the primary antibodies with an IgG1-negative control antibody (Dako).
The tissue sections were rinsed in 0.1 M PBS for 20 minutes and blocked in protein block (Dako) for one hour at room temperature after quenching endogenous peroxidase activity by exposing slides to 3% H2O2 and 10% methanol for 20 minutes. They were then incubated with anti-TonEBP antibody diluted 1 : 5,000 in 0.1 M PBS for 12 hours at 4℃. The slides were washed in 0.1 M PBS and incubated for 1 hour with anti-rabbit antibodies conjugated to horseradish peroxidase (Southern Biotech, Birmingham, AL, USA) in 0.1 M PBS. The slides were colored with DAB/H2O2 solution (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, each slide was incubated in a similar way by using anti-cytokeratin 7 antibody and anti-vimentin antibody as a primary antibody (diluted 1 : 5,000, 1 : 1,000). The slides were washed in 0.1 M PBS and incubated for 1 hour with anti-mouse antibodies conjugated to horseradish peroxidase (Southern Biotech) in 0.1 M PBS. The slides were colored with vector SG substrate kit (Vector Laboratories, Burlingame, CA, USA). Sections were viewed and photographed under a light microscope (Zeiss Axioskop 2; Carl Zeiss, Jena, Germany).
The 4% paraformaldehyde-fixed mouse placentas were embedded in paraffin and 4-µm sections were prepared. Digoxigenin-labeled antisense SMIT riboprobe and sense SMIT riboprobe were prepared with using DIG RNA labeling kit (Boehringer Mannheim, Indianapolis, IN, USA) using rat TonEBP cDNA corresponding to nucleotides 4,026 to 4,319 of the human TonEBP cDNA. After deparaffinization and rehydration, the sections were treated in 0.02 N HCl for 20 minutes and 0.01 N HCl with 0.4% pepsin for 10 minutes at room temperature. The sections were rinsed with diethyl pyrocarbonate treated PBS and dehydrated with graded ethanol series and dried. Prehybridization was performed for 3 hours at 53℃ in 50% formamide, 4× SSC, 10% dextran sulfate, 1× Denhardt's solution, and 1 mg/ml salmon sperm DNA. Hybridization was followed for 15 hours in the same solution with digoxigenin-labeled SMIT antisense riboprobe (400 ng/ml) instead of salmon sperm DNA. Same procedure with SMIT sense riboprobe (400 ng/ml) was performed for a negative control. The sections were washed and incubated with anti-digoxigenin antibody conjugated with alkaline phosphatase, and histochemical detection was then performed using nitrobluetetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Boehringer Mannheim).
TonEBP mRNA is most abundant in placenta [6]. We performed immunohistochemistry for TonEBP in normal human full term placenta and confirmed expression of TonEBP. TonEBP staining was strong in the nucleus of villous trophoblasts. Some endothelial cells and other connective cells were weakly stained (Table 1, Fig. 1A). TonEBP was also detectable in a cell mass of basal plate (Fig. 1B). Localization of TonEBP was confirmed with various cell markers, cytokeratin 7 and vimentin in serial section (3 µm) of paraffin block. The TonEBP positive cell mass in basal plate colocalized with cytokeratin 7 but not with vimentin (Fig. 1C, D). Cytokeratin 7 is a marker for extravillous trophoblasts [14]. TonEBP immunoreactivity was mainly in villous and extravillous trophoblasts. The placenta stained with preimmune serum did not produce significant staining (Fig. 1E).
Expression of SMIT mRNA was examined by in situ hybridization of the antisense riboprobe. The sense riboprobe did not produce significant hybridization (Fig. 2C). SMIT mRNA was strongly detected in the cytoplasm of trophoblasts and weakly in endothelial cells (Table 1, Fig. 2A, B). The localization of SMIT was similar to that of TonEBP.
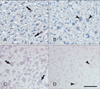
To make clear the relation between TonEBP and SMIT, immunohistochemistry of TonEBP and in situ hybridization of SMIT was carried out in TonEBP knockout mouse placenta. Both TonEBP and SMIT were showed positive signal in labyrinthine trophoblasts of wild type mouse (Fig. 3A, C). As expected, TonEBP expression was not detected in TonEBP knockout mouse and SMIT mRNA was also absent in the TonEBP knockout mouse (Fig. 3B, D).
This is the first study describing localization of TonEBP and SMIT in the placenta. We can assume that antibody and riboprobe worked specifically because the negative control didn't show any signal. TonEBP is present mainly in villous and extravillous trophoblasts, weakly in endothelial cell. The distribution of SMIT is similar to TonEBP expression, and absence of TonEBP leads to deficiency of SMIT. The conclusion of these findings is that TonEBP regulates transcription of SMIT in placenta. What is their function in placenta?
In the central nervous system, the trapping of ~2-15 mM levels of Ins within a neuron through active transport, restricted efflux, and relatively high extracellular Ins levels may be essential to its homeostasis [10]. Highly expressed SMIT in central nervous system plays an important role in maintenance of concentration gradient of Ins [13, 15]. Berry et al. [11] found that SMIT knockout mouse dies immediately after birth due to central apnea. The cause of death is defective brainstem control of breathing: the pre-Botzinger respiratory control center discharges abnormal electrical rhythm [11]. But SMIT knockout mice are rescued by feeding 1% inositol solution during pregnancy [12]. The TonEBP knockout mice apparently display the same phenotype as SMIT knockout mice (data not shown).
To transport nutrition from mother to fetus, it passes through trophoblasts and endothelial cells in placenta. TonEBP and SMIT are abundant in placenta, and their location is mainly trophoblasts and endothelial cells. TonEBP in these cells regulate transcription of SMIT, and SMIT may play an important role in transporting Ins from maternal blood to fetal blood vessels. In conclusion, TonEBP and SMIT transport Ins to fetus in placenta. Further studies will be needed to clarify the function of TonEBP and SMIT in placenta.
Figures and Tables
Fig. 1
Immunohistochemical localization of tonicity-responsive enhancer binding protein (TonEBP) in human placenta. (A) Nuclear staining of TonEBP was mainly in the villous trophoblasts (arrows). Some endothelial cells and connective cells were weakly stained. (B) TonEBP stained cell mass was showed in the basal plate (arrows). (C) Nuclear TonEBP stained cells (brown) were colocalized with cytokeratin 7 (blue). (D) Nuclear TonEBP stained cells (brown) were not colocalized with vimentin (blue). Serial 3 µm section of (C). (E) Negative control showed no labeling. Scale bars=50 µm (A-E).

Fig. 2
In situ hybridization of sodium-myo-inositol cotransporter (SMIT) in human placenta. (A, B) Cytoplasmic staining of SMIT antisense riboprobe was mainly in the villous trophoblast (arrows). Some endothelial cells and connective cells were weakly stained. (C) As a negative control, no specific staining of SMIT sense riboprobe was seen. Scale bars=50 µm (A-C).
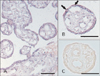
Fig. 3
Immunohistochemical localization of tonicity-responsive enhancer binding protein (TonEBP) and in situ hybridization of sodium-myo-inositol cotransporter (SMIT) in TonEBP knockout mouse. (A) Nucleus of labyrinthin trophoblasts were stained for TonEBP immunohistochemistry in wild type mouse placenta (arrows). (B) No specific staining of TonEBP was seen in TonEBP knockout mouse placenta (arrowheads). (C) Cytoplasm of labyrinthin trophoblasts were stained for SMIT in situ hybridization in wild type mouse placenta (arrows). (D) No specific staining of SMIT was seen in TonEBP knockout mouse placenta (arrowheads). Scale bars=50 µm (A-D).
References
1. Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999. 96:2538–2542.
2. Na KY, Woo SK, Lee SD, Kwon HM. Silencing of TonEBP/NFAT5 transcriptional activator by RNA interference. J Am Soc Nephrol. 2003. 14:283–288.
3. Woo SK, Lee SD, Kwon HM. TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 2002. 444:579–585.
4. Dahl SC, Handler JS, Kwon HM. Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol Cell Physiol. 2001. 280:C248–C253.
5. Trama J, Go WY, Ho SN. The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol. 2002. 169:5477–5488.
6. Trama J, Lu Q, Hawley RG, Ho SN. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J Immunol. 2000. 165:4884–4894.
7. Dawson RM, Freinkel N. The distribution of free mesoinositol in mammalian tissues, including some observations on the lactating rat. Biochem J. 1961. 78:606–610.
8. Stokes CE, Gillon KR, Hawthorne JN. Free and total lipid myo-inositol concentrations decrease with age in human brain. Biochim Biophys Acta. 1983. 753:136–138.
9. Godfrey DA, Hallcher LM, Laird MH, Matschinsky FM, Sherman WR. Distribution of myo-inositol in the cat cochlear nucleus. J Neurochem. 1982. 38:939–947.
10. Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002. 82:736–754.
11. Berry GT, Wu S, Buccafusca R, Ren J, Gonzales LW, Ballard PL, Golden JA, Stevens MJ, Greer JJ. Loss of murine Na+/myo-inositol cotransporter leads to brain myo-inositol depletion and central apnea. J Biol Chem. 2003. 278:18297–18302.
12. Buccafusca R, Venditti CP, Kenyon LC, Johanson RA, Van Bockstaele E, Ren J, Pagliardini S, Minarcik J, Golden JA, Coady MJ, Greer JJ, Berry GT. Characterization of the null murine sodium/myo-inositol cotransporter 1 (Smit1 or Slc5a3) phenotype: myo-inositol rescue is independent of expression of its cognate mitochondrial ribosomal protein subunit 6 (Mrps6) gene and of phosphatidylinositol levels in neonatal brain. Mol Genet Metab. 2008. 95:81–95.
13. Berry GT, Mallee JJ, Kwon HM, Rim JS, Mulla WR, Muenke M, Spinner NB. The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): molecular cloning and localization to chromosome 21. Genomics. 1995. 25:507–513.
14. Benirschke K, Kaufmann P, Baergen RN. Pathology of the human placenta. 2006. 5th ed. New York: Springer;24–29.
15. Guo W, Shimada S, Tajiri H, Yamauchi A, Yamashita T, Okada S, Tohyama M. Developmental regulation of Na+ / myo-inositol cotransporter gene expression. Brain Res Mol Brain Res. 1997. 51:91–96.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download