Abstract
The infrared (IR) receptors in the pit organ of crotaline snakes are very sensitive to temperature. The sensitivity to IR radiation is much greater in crotaline snakes than in boid snakes because they have a thermosensitive membrane suspended in a pair of pits that comprise the pit organ. The vasculature of the pit membrane, which is located near IR-sensitive terminal nerve masses, the IR receptors, supplies the blood necessary to provide cooling and the energy and oxygen that the IR receptors require. The ophthalmic and maxillary branches of the trigeminal nerve innervate the pit membrane. In crotaline snakes, the trigeminal ganglion (TG) is divided into the ophthalmic and maxillomandibular ganglia; a prominent septum further separates the two divisions of the maxillomandibular ganglion. The TG neurons in the ophthalmic ganglion and the maxillary division of the maxillomandibular ganglion relay IR sensation to the brain. This article reviews the IR-sensitive pit organ and trigeminal sensory system structures in crotaline snakes.
Animals can detect temperature changes in their environment with special thermoreceptors, which aid in hunting, feeding, and survival in many organisms, including crotaline and boid snakes [1], vampire bats [2, 3], fire-seeking beetles [4, 5], certain butterflies [6, 7], and blood-sucking bugs [5, 8]. Crotaline and boid snakes have specialized infrared (IR) receptors in pit organs that enable them to detect, locate, and apprehend prey when combined with other sensory systems [1]. IR sensitivity in crotaline and boid snakes has likely evolved from the somatic sensory system, which evolved to sense IR radiation, similar to the vision sensation mechanism [9]. IR radiation sensitivity is much greater in crotaline and boid snakes than in other thermosensitive animals, and crotaline snakes have more sensitive IR radiation receptors than boid snakes [10].
This review describes the structures of the IR-sensitive pit organ and trigeminal sensory systems, which connect IR sensation to the brain, in crotaline snakes.
Two snake families have IR receptors: Boidae (boid snakes) and the subfamily Crotalinae in the Viperidae (crotaline snakes). Crotaline snakes have pits comprising the pit organ located on both sides of their face roughly midway between the nostril and the eye [1, 11]. The pit organ of crotaline snakes consists of a 1-5-mm-wide cavity, several times the diameter of the nostril (Fig. 1A) [11]. A thin pit membrane is suspended inside the cavity between the inner and outer chambers of a pair of pits in the loreal region [11]. The outer chamber opens widely to the exterior, whereas the inner cavity interacts with the external air via a sphincter-controlled pore rostral to the eye [11]. Fig. 1B shows a diagram of a crotaline pit organ in cross-section. In comparison, boid snakes have receptors in their labial scales, either without specialized structures or in the fundus of specialized labial pits (Fig. 1C) [1, 10, 12].
IR radiation sensitivity is much greater in crotaline snakes than in boid snakes [10, 12, 13], and IR sensation in crotaline snakes requires lower temperature changes and energy thresholds of 0.003℃ and 10.75 µW/cm2, respectively, and has a greater detection distance (66.3 cm) than that in boid snakes [10, 13], due to receptor depth and pit organ anatomy [9]. In the crotaline snake Trimeresurus flavoviridis, the pit organ membrane is approximately 15 µm thick [11] and is surrounded by air on both sides, preventing heat loss by conduction to surrounding tissues and allowing most of the IR radiation incident on the pit to heat the membrane [11]. Approximately 7,000 IR-sensitive trigeminal sensory axon endings are distributed throughout the membrane, which excite their nerve fibers when warmed [9]. Similar heat-sensitive nerve endings cover the bottom of each pit in boid snakes [9], because their receptors are not suspended, as in crotaline snakes. Unlike the receptors in the floor of the labial pits or scales, the pit membrane of crotaline pit organs is highly innervated and has a dense capillary bed that projects throughout the network of mitochondria-rich terminal nerve masses (TNMs), supplying blood for cooling and providing energy and oxygen to the TNMs (Fig. 2A) [9, 14]. The pit membrane vasculature is finer, flatter, and more convoluted than that of other sensory organs, including the skin and retina [14]. Scanning electron microscopy has revealed the three-dimensional morphology of the fundamental structures that comprise the IR receptor system in crotaline snake pit membranes (Fig. 2). The TNMs are arranged in one layer directly beneath the outer epithelium of the pit membrane (Fig. 2A, B). A group of myelinated nerve fibers branch to innervate the TNMs at a point farthest from the outer epithelium, below the TNMs and capillary network (Fig. 2A, C). The intimate apposition of the blood capillaries with the TNMs and their feeder nerve fibers is shown in Fig. 2D. Viewed from the inner chamber, the myelinated fibers are beneath the TNMs and become unmyelinated as they bend upward to form the array of sensors (Fig. 2E).
In crotaline snakes, the pit organ has unusual vasculature and numerous IR-sensitive TNMs [11, 14, 15]. Due to the extensive nature of the vasculature and its proximity to the TNMs [14, 15], chemicals administered via the blood reach the TNMs. This morphology has been examined by studying the IR receptor response to vasoactive chemicals, which occurs either by a vasoactive effect on the pit membrane vasculature or by a chemical effect on the IR receptor thermoreceptor channels, such as the transient receptor potential (TRP) vanilloid family [16-18]. Gracheva et al. [19] and Panzano et al. [20] found that pit organs respond to temperature using the heat-activated cation channel TRP ankyrin 1 (TRPA1). They suggested using TRPA1 and other TRP channels as new genetic and physiological markers to delineate evolutionary relationships between vertebrates and invertebrates. Further studies are required to confirm if IR receptors have other TRP channels.
In mammals, the trigeminal nerve connects sensations including touch, pressure, pain, and temperature from the facial, maxillary, and mandibular regions to the brain. Crotaline snake pit organs are also innervated by the trigeminal nerve, which contains a very pure population of unimodal warm nerve fibers [1]. Three branches of the trigeminal nerve innervate the pit membrane: one ramus ophthalmicus branch and two ramus maxillaries branches. These trigeminal ganglion (TG) neuron fibers connect ipsilaterally in the lateral descending nucleus of the medulla oblongata [21, 22]. This system is independent of the common trigeminal sensory system found in these snakes and other vertebrates. Other reports have examined the IR afferent system, including the nucleus reticularis caloris [23] and optic tectum [21, 24].
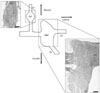
In amphibians and mammals, the ophthalmic and maxillomandibular ganglia are fused to form one complex trigeminal or semilunar ganglion. Conversely, the two ganglia remain separate in most reptiles [25]. A prominent septum further separates the two divisions of the maxillomandibular ganglion in crotaline snakes [26]. In a horseradish peroxidase (HRP) tracing study, HRP cell labeling from the pit organ to the TG revealed HRP-positive IR-sensory TG neurons in the ophthalmic ganglion and the maxillary division of the maxillomandibular ganglion but not in the mandibular division [27]. Fig. 3 shows a diagram of the TG structure in crotaline snakes. Based on its specialized structure, several immunohistochemical studies have described the distribution of various neuronal signals in the TG, including calcitonin gene-related peptide [28], neuronal and endothelial nitric oxide synthases [29, 30], and protein kinase C delta [31].
Some animals and insects have thermoreceptors, which aid hunting, feeding, and survival. IR radiation sensitivity is much greater in crotaline and boid snakes than in other thermosensitive animals. Furthermore, crotaline snakes have a more sensitive IR receptor than boid snakes because they have a thin thermosensitive membrane suspended between a pair of pits, located in the loreal region, which comprises the pit organ. The pit membrane vasculature, located near IR-sensitive terminal nerve masses, the IR receptors, supplies blood for cooling and provides the energy and oxygen required by the IR receptors. The ophthalmic and maxillary branches of the TG nerves innervate the pit membrane. In crotaline snakes, the TG is separated into the ophthalmic and maxillomandibular ganglia and a prominent septum further separates the two divisions of the maxillomandibular ganglion. TG ganglion neurons in the ophthalmic ganglion and the maxillary division of the maxillomandibular ganglion, but not in the mandibular division, connect IR sensation to the brain. Further studies are required to expand what is known about the thermosensitive TRP channels in the crotaline pit organ IR receptor.
Figures and Tables
Fig. 1
The pit organ of the crotaline snake Trimeresurus flavoviridis. (A) Crotaline snakes have a pair of pit organs, which are infrared (IR) receptors, one located on each side of the face roughly midway between the nostril and the eye. (B) A cross-section of the pit organ in crotaline snakes. (C) Cross-sections of the two types of IR receptor organs in boid snakes. IR receptors are located beneath the surface of the labial scales and the fundus of the pits. E, eye; N, nostril; PO, pit organ.
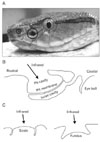
Fig. 2
Scanning electron micrographs of the pit membrane in the crotaline snake Trimeresurus flavoviridis. (A) A cross-section of the crotaline pit membrane. (B) TNMs viewed from above after stripping the OEC (inset). Individual TNMs exhibit a network of trenches, demarcating the surface into many incomplete compartments. (C) TNMs and MF viewed from beneath after stripping the IEC (inset). The nerves entering (left) the pit membrane are unbranched MFs, with a diameter of 3.53±0.49 µm (n=10), and they course beneath the TNMs. (D) A micrograph of the pit membrane viewed from above after stripping away the OEC and some TNMs (inset). A UF terminates on a BV. The diameter was 0.21±0.05 µm (n=10). (E) A lateral view of a TNM with an axon (inset). White arrow indicates where the MF loses its sheath. BV, blood vessel; IEC, inner epithelium and connective tissue; MF, myelinated nerve fiber; OEC, outer epithelium and connective tissue; TNM, terminal nerve mass; UF, unmyelinated nerve fiber. Scale bars=10 µm (B-D), 5 µm (E).
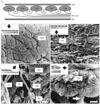
Fig. 3
A schematic diagram and the histological structure of a horizontal section of a trigeminal ganglion in the crotaline snake Trimeresurus flavoviridis. OP, ophthalmic ganglion; MM, maxilla-mandibular ganglion; V1, ophthalmic nerve; V2, maxillary nerve; V3, mandibular nerve. Hematoxylin and eosin staining. Scale bars=200 µm.
Acknowledgements
The author thanks Dr. S. Terashima and Dr. T. Shin for critical reading and comments on the manuscript.
References
1. Bullock TH, Diecke FP. Properties of an infra-red receptor. J Physiol. 1956. 134:47–87.
2. Kishida R, Goris RC, Terashima S, Dubbeldam JL. A suspected infrared-recipient nucleus in the brainstem of the vampire bat, Desmodus rotundus. Brain Res. 1984. 322:351–355.
3. Kürten L, Schmidt U. Thermoperception in the common vampire bat (Desmodus rotundus). J Comp Physiol A. 1982. 146:223–228.
4. Evans WG. Infra-red receptors in Melanophila acuminata degeer. Nature. 1964. 202:211.
5. Schmitz H, Bleckmann H. The photomechanic infrared receptor for the detection of forest fires in the beetle Melanophila acuminata (Coleoptera: Buprestidae). J Comp Physiol A. 1998. 182:647–657.
6. Schmitz H, Wasserthal LT. Antennal thermoreceptors and wing-thermosensitivity of heliotherm butterflies: their possible role in thermoregulatory behavior. J Insect Physiol. 1993. 39:1007–1019.
7. Schmitz H. Thermal characterization of butterfly wings 1. Absorption in relation to different color, surface structure and basking type. J Therm Biol. 1994. 19:403–412.
8. Lazzari CR, Nunez JA. The response to radiant heat and the estimation of the temperature of distant sources in Triatoma infestans. J Insect Physiol. 1989. 35:525–529.
9. Newman EA, Hartline PH. The infrared "vision" of snakes. Sci Am. 1982. 246:116–127.
10. Campbell AL, Naik RR, Sowards L, Stone MO. Biological infrared imaging and sensing. Micron. 2002. 33:211–225.
11. Terashima SI, Goris RC, Katsuki Y. Structure of warm fiber terminals in the pit membrane of vipers. J Ultrastruct Res. 1970. 31:494–506.
12. Barrett R, Maderson PF, Meszler RM. Gans C, editor. The pit organs of snakes. Biology of Reptilia. 1970. London: Academic Press;277–314.
13. de Cock Buning T. Thermal sensitivity as a specialization for prey capture and feeding in snakes. Am Zool. 1983. 23:363–375.
14. Amemiya F, Nakano M, Goris RC, Kadota T, Atobe Y, Funakoshi K, Hibiya K, Kishida R. Microvasculature of crotaline snake pit organs: possible function as a heat exchange mechanism. Anat Rec. 1999. 254:107–115.
15. Amemiya F, Ushiki T, Goris RC, Atobe Y, Kusunoki T. Ultrastructure of the crotaline snake infrared pit receptors: SEM confirmation of TEM findings. Anat Rec. 1996. 246:135–146.
16. Moon C, Terashima S. Response of the infrared receptors of a crotaline snake to ethanol. Neurosci Lett. 2002. 334:29–32.
17. Moon C. An investigation of the effects of ruthenium red, nitric oxide and endothelin-1 on infrared receptor activity in a crotaline snake. Neuroscience. 2004. 124:913–918.
18. Moon C, Terashima S, Yasuzumi F, Shin T. Snake infrared receptors respond to dimethylsulfoxide in the blood stream. Cell Mol Neurobiol. 2004. 24:749–756.
19. Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sánchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature. 2010. 464:1006–1011.
20. Panzano VC, Kang K, Garrity PA. Infrared snake eyes: TRPA1 and the thermal sensitivity of the snake pit organ. Sci Signal. 2010. 3:pe22.
21. Gruberg ER, Kicliter E, Newman EA, Kass L, Hartline PH. Connections of the tectum of the rattlesnake Crotalus viridis: an HRP study. J Comp Neurol. 1979. 188:31–41.
22. Molenaar GJ, Fizaan-Oostveen JL. Ascending projections from the lateral descending and common sensory trigeminal nuclei in python. J Comp Neurol. 1980. 189:555–572.
23. Kishida R, Amemiya F, Kusunoki T, Terashima S. A new tectal afferent nucleus of the infrared sensory system in the medulla oblongata of Crotaline snakes. Brain Res. 1980. 195:271–279.
24. Newman EA, Gruberg ER, Hartline PH. The infrared trigemino-tectal pathway in the rattlesnake and in the python. J Comp Neurol. 1980. 191:465–477.
25. Stark D. Gans C, Northcutt RC, Ulinski P, editors. Cranio-cerebral relations in recent reptiles. Biology of the Reptilia. 1979. New York: Academic Press;1–38.
26. Molenaar GJ. The sensory trigeminal system of a snake in the possession of infrared receptors. I. The sensory trigeminal nuclei. J Comp Neurol. 1978. 179:123–135.
27. Kishida R, Terashima SI, Goris RC, Kusunoki T. Infrared sensory neurons in the trigeminal ganglia of crotaline snakes: transganglionic HRP transport. Brain Res. 1982. 241:3–10.
28. Sekitani-Kumagai M, Kadota T, Goris RC, Kusunoki T, Terashima S. Calcitonin gene-related peptide immunoreactivity in the trigeminal ganglion of Trimeresurus flavoviridis. Neurosci Res. 1995. 22:287–295.
29. Moon C, Terashima S, Ahn M, Kang J, Shin T. Immunohistochemical analysis of neuronal nitric oxide synthase in the trigeminal ganglia of the crotaline snake Trimeresurus flavoviridis. Neurosci Lett. 2002. 319:21–24.
30. Moon C, Terashima S, Shin T. Immunohistochemical study of endothelial nitric oxide synthase in the trigeminal ganglia of a crotaline snake Trimeresurus flavoviridis. J Vet Med Sci. 2004. 66:1007–1009.
31. Moon C, Terashima S, Shin T. Immunohistochemical localization of the delta subspecies of protein kinase C in the trigeminal sensory system of Trimeresurus flavoviridis, an infrared-sensitive snake. Neurosci Lett. 2003. 338:233–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download