Abstract
Myelinated Schwann cells in the peripheral nervous system express the p75 nerve growth factor receptor (p75NGFR) as a consequence of Schwann cell dedifferentiation during Wallerian degeneration. p75NGFR has been implicated in the remyelination of regenerating nerves. Although many studies have shown various mechanisms underlying Schwann cell dedifferentiation, the molecular mechanism contributing to the re-expression of p75NGFR in differentiated Schwann cells is largely unknown. In the present study, we found that lysosomes were transiently activated in Schwann cells after nerve injury and that the inhibition of lysosomal activation by chloroquine or lysosomal acidification inhibitors prevented p75NGFR expression at the mRNA transcriptional level in an ex vivo Wallerian degeneration model. Lysosomal acidification inhibitors suppressed demyelination, but not axonal degeneration, thereby suggesting that demyelination mediated by lysosomes may be an important signal for inducing p75NGFR expression. Tumor necrosis factor-α (TNF-α) has been suggested to be involved in regulating p75NGFR expression in Schwann cells. In this study, we found that removing TNF-α in vivo did not significantly suppress the induction of both lysosomes and p75NGFR. Thus, these findings suggest that lysosomal activation is tightly correlated with the induction of p75NGFR in demyelinating Schwann cells during Wallerian degeneration.
Wallerian degeneration of nerves occurs in the distal segment of lesion sites after injury. Both Schwann cells and macrophages participate in the removal of myelin in degenerating peripheral nerves [1]. During myelin removal in Schwann cells, the cells begin to acquire several macrophage-like phenotypes [1]. The galactose-specific lectin, MAC-2, and the A1 antigen, a macrophage marker, are strongly expressed in Schwann cells during the early stages of Wallerian degeneration, and inhibition of MAC-2 prevents myelin removal by Schwann cells [2]. Furthermore, demyelination mediated through Schwann cells is enhanced by the lysosomal pathways [3, 4]. Lysosomes contain many hydrolytic enzymes that participate in degrading myelin lipids and proteins. Studies utilizing enzymatic histochemistry have reported that the number of lysosomes and the activity of lysosomal enzymes increase in Schwann cells within 1 week after injury [4, 5]. We recently reported that the protein levels of lysosomal associated membrane protein 1 (LAMP1), a lysosomal membrane marker, increase dramatically in sciatic nerves following injury [6].
Axonal signaling molecules such as neuregulin and neurotrophic factors regulate remyelination by Schwann cells during peripheral nerve regeneration [7-10]. A growing body of evidence suggests that signal transduction mediated by nerve growth factor (NGF) and brain-derived neurotrophic factor via the p75 NGF receptor (p75NGFR) plays a role in the myelination process [8, 11]. p75NGFR is normally expressed in non-myelinated Schwann cells of uninjured nerves, but peripheral nerve injury rapidly induces p75NGFR in demyelinating myelinated Schwann cells [12, 13]. p75NGFR-null mice exhibit impaired remyelination after sciatic nerve injury, thereby suggesting a role for p75NGFR in remyelination in vivo [14, 15]. Derepression through the loss of axonal signals may result in p75NGFR induction in demyelinating Schwann cells after injury [16]. However, the mechanism by which axonal injury regulates p75NGFR induction in Schwann cells is not well understood. In addition to the loss of axonal signals, demyelination after nerve injury may also involve p75NGFR expression in Schwann cells. For example, the demyelinating agent, lysolecithin, induces p75NGFR expression in Schwann cells [17]. Furthermore, several in vitro studies have shown the involvement of tumor necrosis factor-α (TNF-α) in p75NGFR expression in Schwann cells [18-20]. In the present study, we examined whether lysosomal activation, which is a well-characterized mediator of demyelination, is associated with p75NGFR induction in Schwann cells.
The adult mice (C57BL6) sciatic nerve crush injury model was used as previously described [21, 22]. All procedures were performed according to protocols approved by the Dong-A University committee on animal research, which followed the guidelines for animal experiments established by the Korean Academy of Medical Sciences. Briefly, the sciatic nerve was exposed at the mid-thigh level and was crushed twice in succession with fine forceps under anesthesia with a mixture of 10% ketamine and Rompun (Bayer, Leverkusen, Germany). After recovery, mice were sacrificed with a high dose of 10% ketamine, and nerves were removed, fixed with 4% paraformaldehyde (PFA) and cryoprotected overnight. Frozen sections (10-µm thick) were prepared with a cryostat (CM3050, Leica, Solms, Germany) or nerves were teased under a stereomicroscope. The teased nerve fibers were mounted on a slide, dried, and maintained at -70℃.
Sciatic nerve explants were cultured for in vitro Wallerian degeneration as previously reported [6]. Sciatic nerves from adult mice were removed, and connective tissues surrounding the nerves were carefully detached in calcium/magnesium-free Hank's buffered solution. Sciatic nerves were longitudinally cut into two or three nerve explants and were then cut into small, 3-mm long explants. These explants were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% heat-inactivated fetal bovine serum at 37℃ with 5% CO2 for the indicated time. After culture, the nerve explants were fixed with PFA for 6-12 hours and teased into a single or several nerve fibers.
A myelin ovoid was defined as an ovoid fragmented myelin sheath that was completely separated from the adjacent myelin sheath. The number of myelin ovoids was counted in medium to large sized-teased nerve fibers that were approximately 200 µm in length (≤100 nerve fibers/group) under an Axiophot upright microscope equipped with differential interference contrast (DIC) filters (Carl Zeiss, Oberkochen, Germany).
For Western blot analysis, the distal side of the injured nerves was harvested and lysed in modified radioimmune precipitation assay lysis buffer (150 mM NaCl, 1% Nonidet P-40, 1 mM ethylenediaminetetraacetic acid, 0.5% deoxycholic acid, 2 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 5 mM benzamidine, 1 mM sodium orthovanadate, and 1× protease inhibitor cocktail [Roche, Indianapolis, IN, USA]). The lysates were centrifuged at 8,000 ×g for 10 minutes at 4℃, and the supernatant was collected. The protein concentration in each sample was analyzed by the Bradford method. Protein (25-35 µg) was separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). After blocking with 0.1% Tween-20 and 5% nonfat dry milk in Tris-buffered saline (TBS, 25 mM Tris-HCl pH 7.5, 140 mM NaCl) at room temperature for 1 hour, the membrane was incubated with primary antibodies (1 : 500-1,000) in TBS with Tween 20 (TBST) containing 2% nonfat dry milk at 4℃ overnight. After three 15-minute washes with TBST, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1 : 3,000) for 1 hour at room temperature. Signals were detected using the Enhanced Chemiluminescence System (Amersham Biosciences). X-ray film was scanned using an HP scanner and analyzed with the LAS image analysis system for quantification (Fujifilm, Tokyo, Japan).
The teased nerve fibers on slides were blocked with phosphate buffered saline (PBS) containing 0.2% Triton X-100 and 2% bovine serum albumin for 1 hour for immunofluorescent staining. The nerve fibers were then incubated with primary antibody (1 : 1,000) for 16 hours at 4℃ and washed three times with PBS. Next, the slides were incubated with Cy3- or Alexa 488-conjugated secondary antibody (1 : 800, Jackson Immunoresearch Laboratories, West Grove, PA, USA) for 3 hours at room temperature. The sections were then washed three times with PBS, and then mounted with coverslips on glass slides using a mounting medium (Gelmount, Biomeda Co., Foster City, CA, USA) and viewed using a laser confocal microscope (LSM510, Carl Zeiss).
Total RNA was extracted from sciatic nerve explants using the Ultraspec RNA Isolation Kit (Biotecx, Houston, TX, USA) for the reverse transcription-polymerase chain reaction (RT-PCR). For cDNA synthesis, 500 ng of total RNA was used with ready-to-go-your-prime first strand beads (Amersham Biosciences) using oligo(dT) primers, as indicated by the manufacturer. The following primers were used to detect p75NGFR and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in the sciatic nerve. p75NGFR forward primer, 5'-CCCTCAAGGGTGATGGCAACCTCT-3' and reverse primer, 5'-TGTCAGCTCTCTGGATGCGTCGC-3'; GAPDH forward primer, 5'-TGCCGCCTGGAGAAACCTGC-3' and reverse primer, 5'-TGAGAGCAATGCCAGCCCCA-3'. After an initial step at 94℃ for 5 minutes, 28 cycling conditions were carried out at 94℃ for 1 minute, 57℃ for 45 seconds, and 72℃ for 1 minute before a final extension period of 5 minutes at 72℃. To check that the product was accumulating in a linear manner, 1/10 of each reaction was electrophoresed at five cycle intervals on a 1.5% agarose gel stained with ethidium bromide.
TNF-α knockout mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Genomic DNA from the mouse tail was used for PCR genotyping analysis. The primers were as follows: TNF-α common forward primer, 5'-TAGCCAGGAGGGAGAACAGA-3'; TNF-α wild-type reverse primer, 5'-AGTGCCTCTTCTGCCAGTTC-3'; TNF-α mutant reverse primer, 5'-CGTTGGCTACCCGTGATATT-3'. The PCR cycling conditions were 94℃ for 3 minutes, followed by 35 cycles of 94℃ for 30 seconds, 62℃ for 1 minute, 72℃ for 1 minute, and a final extension step at 72℃ for 2 minutes.
We previously showed that LAMP1 expression increases in sciatic nerves following injury using Western blot analysis [6]. In the present study, we examined the localization of lysosomes in Schwann cells using double immunostaining against LAMP1 and S100 (a Schwann cell marker) (Fig. 1). Confocal laser microscopy showed that little LAMP1 immunoreactivity was localized to perinuclear areas in control teased nerve fibers (Fig. 1A). At 3 days after axotomy, the distal regions of the injured sciatic nerves showed intense LAMP1 staining in the internodal areas. LAMP1 staining was colocalized with S100 staining, indicating that early LAMP1 induction originated from Schwann cells (Fig. 1B). At 3 days after injury, the degenerating myelin ovoids were surrounded by intense LAMP1 staining (Fig. 1B), suggesting a possible role for lysosomes in myelin degradation. Thus, we compared the immunofluorescent staining of myelin basic protein (MBP, as a myelin marker) and LAMP1. Immunofluorescent staining against MBP revealed that the extent of myelin degradation was heterogeneous 3 days after injury. MBP staining showed clump-like patterns within myelin ovoids, which were surrounded by LAMP1 staining. In contrast, regions with relatively intact MBP staining in the myelin sheath were devoid of a LAMP1 signal (Fig. 1B), suggesting a role for lysosomes in myelin degradation.
The increase in the number of lysosomes as demonstrated by LAMP1 staining in Schwann cells was transient. At 7 days after injury, LAMP1 immunoreactivity was significantly downregulated in the internodal areas where myelin debris had been completely removed (asterisks in Fig. 1C). Instead, many macrophages, which were labeled with an antibody to CD68, were observed within or outside endoneurial tubes that expressed high levels of LAMP1 (arrows in Fig. 1C). At 3 weeks after injury, the remyelinated fibers, as demonstrated by laminin or MBP immunostaining, were negative for LAMP1 staining (Fig. 1D). However, strong LAMP1-positive macrophages were still found outside these remyelinating fibers. Thus, it may be possible that lysosomal activation in Schwann cells is transient and that macrophages participate in the lysosomal degradation of myelin during the late phase of Wallerian degeneration.
Lysosomal activation may play a role in the demyelination process. Many lysosomal enzymes rely on an acidic pH for optimal activation. We examined whether lysosomal acidification inhibitors affected demyelination using ex vivo explants cultures, which have been previously established as a useful in vitro model for Wallerian degeneration [6]. First, the effect of lysosomal acidification inhibitors on myelin ovoid formation, which is an initial sign of demyelination, was examined under DIC microscopy (Fig. 2A, B). Bafilomycin A (BFA) and NH4Cl significantly inhibited myelin ovoid formation at 3 days of culture (3DIV). Chloroquine (CQ) has been used as a lysosomotropic amine to disrupt lysosomal membrane integrity, thereby inhibiting lysosomal activity [23]. Thus, we tested CQ to examine whether lysosomal activation participates in myelin ovoid formation. As shown in Fig. 2, myelin ovoid formation at 3DIV was prevented by CQ, which further suggests that lysosomal acidification is essential for myelin ovoid formation.
Second, we examined the destruction of MBP in the presence or absence of lysosomal acidification inhibitors in explants cultures to show that myelin protein degradation is associated with lysosomal acidification. Western blot analyses showed that MBP levels were downregulated with time in vitro, and that BFA and NH4Cl suppressed the decrease in MBP at 6DIV (Fig. 2C). However, these drugs did not affect LAMP1 induction during in vitro Wallerian degeneration (Fig. 2D). Thus, lysosomal acidification seems to be required for demyelination during Wallerian degeneration.
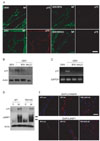
To determine whether lysosomal acidification is necessary for p75NGFR induction, we immunostained teased nerve fibers at 3DIV in the presence or absence of lysosomal acidification inhibitors. The induction of p75NGFR in degenerating nerves was clearly observed at 3DIV, and we found that NH4Cl and BFA significantly prevented p75NGFR induction. Axonal degradation, as demonstrated by neurofilament staining, still occurred even in the presence of the drugs, thereby suggesting that lysosomal acidification plays no role in axonal degeneration (Fig. 3A). Western blot analyses also revealed that these lysosomal acidification inhibitors suppressed p75NGFR induction at 3DIV (Fig. 3B). To determine if the regulation of p75NGFR expression by lysosomal acidification was mediated at the transcriptional level, we examined p75NGFR mRNA expression using RT-PCR. As a result, p75NGFR mRNA expression increased significantly at 3DIV, and this mRNA induction was inhibited by BFA and NH4Cl (Fig. 3C). Taken together, these findings suggest that lysosomal acidification plays an important role in p75NGFR expression in demyelinating Schwann cells at the transcriptional level.
Previous reports have shown that TNF-α regulates gene expression in Schwann cells, including p75NGFR in vitro [18-20, 24]. To directly test whether TNF-α is involved in injury-induced LAMP1 and p75NGFR expression in demyelinating Schwann cells, we examined LAMP1 and p75NGFR induction in the injured sciatic nerve of TNF-α null mice using Western blot analyses and immunofluorescent staining (Fig. 3D, E). In the TNF-α null mice, p75NGFR expression increased in the distal stump of the injured sciatic nerve. Furthermore, LAMP1 induction was not significantly altered in TNF-α knockout mice compared to wild-type mice, suggesting that TNF-α does not play an important role in injury-induced p75NGFR and LAMP1 expression in vivo.
After axonal injury, Schwann cells undergoing Wallerian degeneration and re-express many genes that were expressed during embryonic development. This change in gene expression results in phenotypic changes that transform differentiated Schwann cells into premature Schwann cells, which is called dedifferentiation [25]. Axonal signaling has been suggested as a primary regulating factor for Schwann cell gene expression, including p75NGFR. A loss of axonal contact by injury results in p75NGFR induction in Schwann cells, and its subsequent downregulation is accompanied by axonal regrowth [12, 13]. Consistent with these findings, cultures of adult Schwann cells derived from intact nerves exhibit constitutive induction of p75NGFR [26]. Additionally, neurons suppress p75NGFR expression in Schwann cell co-cultures [16]. Thus, it has been suggested that axonal signals constitutively suppress p75NGFR induction and that the loss of axonal signals from injury results in the induction of p75NGFR. However, how the depression of axonal signals mechanistically regulates p75NGFR expression is not well understood. Our data suggest, for the first time, that lysosomal activation may mediate an intermediate step between the loss of axonal signals and p75NGFR induction. In other words, the loss of axonal signals leads to lysosome activation in demyelinating Schwann cells, and, subsequently, Schwann cells upregulate p75NGFR. Interestingly, the transient activation profiles of lysosomes during Wallerian degeneration are consistent with the role of axonal signals in lysosomal activation. At 7 days after injury, LAMP1 staining in Schwann cells, which had finished myelin degradation, was markedly downregulated. Furthermore, regenerated nerve fibers at 2 and 3 weeks after injury did not show LAMP1 induction. Thus, axonal signals may regulate both lysosomal and p75NGFR induction in Schwann cells.
Quantitative histochemical techniques have demonstrated an increase in the number of lysosomes in demyelinating Schwann cells during Wallerian degeneration [4]. Specifically, these findings have revealed that the activity of several lysosomal enzymes, including acid proteinase, increase in injured nerves, and that these enzymes may participate in myelin degradation. The intralysosomal environment is maintained near pH 4.5 by membrane-bound H-ATPases, and BFA inhibits the H-ATPase and prevents acidification of lysosomes [27]. There is general agreement that lysosomal acidification inhibitors suppress protein degradation in lysosomes, because lysosomal hydrolytic enzymes are optimally active at an acidic pH [28]. In the present study, we found that the inhibition of lysosomal acidification suppressed demyelination. This finding was demonstrated by the changed MBP levels; however, no effect on axonal degeneration was observed. Thus, this finding suggests that lysosome-dependent myelin degradation may participate in p75NGFR induction, and that axonal degeneration may not be sufficient for p75NGFR induction. In accordance with this hypothesis, it has been previously reported that the occurrence of demyelination is associated with p75NGFR expression in many pathological conditions involving the peripheral nervous system. For example, immune-mediated and lysolecithin-induced demyelination is accompanied by changes in p75NGFR expression in peripheral nerves [29]. Patients with hereditary and tellurium-induced neuropathy present with demyelination and exhibit changes in p75NGFR expression [30, 31]. Thus, demyelination induced by lysosomal enzymes might be important for inducing p75NGFR expression.
In contrast to several in vitro studies, which show the role of TNF-α in p75NGFR induction in Schwann cells, TNF-α knockout mice did not show an obvious difference compared to wild-type mice with respect to p75NGFR expression in the sciatic nerves after injury. TNF-α may play a minor role in p75NGFR induction in demyelinating Schwann cells in vivo or compensation by other factors, such as lysosomal activation, may result in the absence of an obvious phenotype in TNF-α knockout mice.
In conclusion, the present study showed that lysosomal activation was essential for p75NGFR induction in demyelinating Schwann cells after peripheral nerve injury. Thus, regulation of lysosomal activity may provide an important strategy for managing Schwann cell dedifferentiation associated with Wallerian degeneration and some peripheral neuropathies.
Figures and Tables
Fig. 1
Transient induction of lysosomal associated membrane protein 1 (LAMP1) in demyelinating Schwann cells. (A) LAMP1 was localized in perinuclear areas (arrows) in control sciatic nerves. (B) Sciatic nerve crush-induced LAMP1 in demyelinating Schwann cells, particularly around myelin ovoids 3 days (3d) after injury. The upper panels are double staining against LAMP1 and S100 (arrow), and the lower panels are double staining against LAMP1 and myelin basic protein (MBP). Asterisk in the middle panels are myelin ovoids demonstrated by differential interference microscopy. Asterisks in the lower panels are MBP-positive clumps surrounded by LAMP1 immunoreactivity. Arrows indicate a LAMP1-negative intact myelin sheath. (C) Downregulation of LAMP1 in demyelinating Schwann cells 7 days (7d) after injury (asterisks). Arrows indicate LAMP1 and CD68-positive macrophages. Macrophages were found inside or outside the endoneurial tube labeled with antibody against laminin (LAM). (D) Three weeks (3W) after injury, remyelinating Schwann cells (labeled with MBP or LAM) were devoid of LAMP1 staining, whereas many LAMP1-positive macrophages were still present around remyelinating Schwann cells. DIC, differential interference contrast. Scale bars=100 µm.
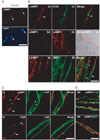
Fig. 2
Lysosomal acidification is required
for demyelination. (A) Differential interference microscopy of teased nerve fibers after 3 days of culture in vitro (3DIV). Scale bar=200 µm. (B) Quantitative result showing myelin ovoid index. (C, D) Western blot analysis showing the levels of myelin basic protein (MBP) (C) and lysosomal associated membrane protein 1 (LAMP1) (D) in cultured nerve explants. "C" indicates uninjured sciatic nerves. CQ, chloroquine; BFA, bafilomycin A.
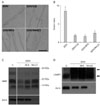
Fig. 3
Lysosomal acidification inhibitors prevent p75 nerve growth factor receptor (p75NGFR) induction. (A) Immunofluorescence microscopy showing p75NGFR expression in teased nerve fibers. Neurofilament (NF) staining showed extensive fragmentation at 3 days culture in vitro (3DIV), regardless of the inhibition of lysosomal acidification. (B) Western blot analysis showing the levels of p75NGFR in cultured nerve explants at 3DIV. (C) Reverse transcription-polymerase chain reaction experiment showing the induction of p75NGFR mRNA in cultured nerve explants at 3 days. (D) Western blot analysis showing the levels of p75NGFR in the sciatic nerves of tumor necrosis factor-α null mice (TNF-KO). (E) Immunofluorescence microscopy showing p75NGFR and lysosomal associated membrane protein 1 (LAMP1) expression in teased nerve fibers 3 days (3d) after injury. "C" indicates uninjured sciatic nerves. BFA, bafilomycin A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 3d, three days after crush; WT, wild-type; Con, control. Scale bars=100 µm.
References
1. Martini R, Fischer S, López-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008. 56:1566–1577.
2. Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994. 14(5 Pt 2):3231–3245.
3. Hallpike JF, Adams CW. Proteolysis and myelin breakdown: a review of recent histochemical and biochemical studies. Histochem J. 1969. 1:559–578.
4. Hallpike JF, Adams CW, Bayliss OB. Histochemistry of myelin. IX. Neutral and acid proteinases in early Wallerian degeneration. Histochem J. 1970. 2:209–218.
5. Weller RO, Mellick RS. Acid phosphatase and lysosome activity in diphtheritic neuropathy and Wallerian degeneration. Br J Exp Pathol. 1966. 47:425–434.
6. Lee HK, Shin YK, Jung J, Seo SY, Baek SY, Park HT. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009. 57:1825–1834.
7. Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992. 119:45–54.
8. Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000. 12:4171–4180.
9. Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008. 22:2970–2980.
10. Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009. 57:1178–1191.
11. Xiao J, Kilpatrick TJ, Murray SS. The role of neurotrophins in the regulation of myelin development. Neurosignals. 2009. 17:265–276.
12. Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987. 104:1623–1631.
13. Taniuchi M, Clark HB, Schweitzer JB, Johnson EM Jr. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988. 8:664–681.
14. Song XY, Zhou FH, Zhong JH, Wu LL, Zhou XF. Knockout of p75(NTR) impairs re-myelination of injured sciatic nerve in mice. J Neurochem. 2006. 96:833–842.
15. Tomita K, Kubo T, Matsuda K, Fujiwara T, Yano K, Winograd JM, Tohyama M, Hosokawa K. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia. 2007. 55:1199–1208.
16. Bolin LM, Shooter EM. Neurons regulate Schwann cell genes by diffusible molecules. J Cell Biol. 1993. 123:237–243.
17. Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993. 45:175–182.
18. Schneider-Schaulies J, Kirchhoff F, Archelos J, Schachner M. Down-regulation of myelin-associated glycoprotein on Schwann cells by interferon-gamma and tumor necrosis factor-alpha affects neurite outgrowth. Neuron. 1991. 7:995–1005.
19. Bonetti B, Valdo P, Stegagno C, Tanel R, Zanusso GL, Ramarli D, Fiorini E, Turazzi S, Carner M, Moretto G. Tumor necrosis factor alpha and human Schwann cells: signalling and phenotype modulation without cell death. J Neuropathol Exp Neurol. 2000. 59:74–84.
20. Lisak RP, Bealmear B, Benjamins JA, Skoff AM. Interferon-gamma, tumor necrosis factor-alpha, and transforming growth factor-beta inhibit cyclic AMP-induced Schwann cell differentiation. Glia. 2001. 36:354–363.
21. Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 2007. 102:686–698.
22. Lee HK, Seo IA, Suh DJ, Hong JI, Yoo YH, Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 2009. 108:776–786.
23. Michihara A, Toda K, Kubo T, Fujiwara Y, Akasaki K, Tsuji H. Disruptive effect of chloroquine on lysosomes in cultured rat hepatocytes. Biol Pharm Bull. 2005. 28:947–951.
24. Boyle K, Azari MF, Cheema SS, Petratos S. TNFalpha mediates Schwann cell death by upregulating p75NTR expression without sustained activation of NFkappaB. Neurobiol Dis. 2005. 20:412–427.
25. Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008. 56:1552–1565.
26. Vroemen M, Weidner N. Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cells from adult peripheral nerve. J Neurosci Methods. 2003. 124:135–143.
27. Nakashima S, Hiraku Y, Tada-Oikawa S, Hishita T, Gabazza EC, Tamaki S, Imoto I, Adachi Y, Kawanishi S. Vacuolar H+-ATPase inhibitor induces apoptosis via lysosomal dysfunction in the human gastric cancer cell line MKN-1. J Biochem. 2003. 134:359–364.
28. Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem. 2002. 383:1827–1831.
29. Stoll G, Li CY, Trapp BD, Griffin JW. Expression of NGF-receptors during immune-mediated and lysolecithin-induced demyelination of the peripheral nervous system. J Neurocytol. 1993. 22:1022–1029.
30. Toews AD, Griffiths IR, Kyriakides E, Goodrum JF, Eckermann CE, Morell P, Thomson CE. Primary demyelination induced by exposure to tellurium alters Schwann cell gene expression: a model for intracellular targeting of NGF receptor. J Neurosci. 1992. 12:3676–3687.
31. Hanemann CO, Gabreëls-Fasten AA, Müller HW, Stoll G. Low affinity NGF receptor expression in CMT1A nerve biopsies of different disease stages. Brain. 1996. 119(Pt 5):1461–1469.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download