Introduction
Radial glia are cells that serve as scaffolding for the radial migration of neuroblasts in the central nervous system (CNS). After neuroblast migration is complete, radial glia are transformed into astrocytes [1-3]. It is generally accepted that vimentin-positive radial glia are astrocyte precursor cells (APCs). Recently, the lineage of astrocytic cells was classified by antigenic phenotyping into four distinct stages in the retina of a rodent: Pax2-positive/vimentin-positive/glial fibrillary acidic protein (GFAP)-negative APCs, Pax2-positive/vimentin-positive/GFAP-positive immature perinatal astrocytes, Pax2-positive/vimentin-negative/GFAP-positive mature perinatal astrocytes, and Pax2-negative/vimentin-negative/GFAP-positive astrocytes [4]. Based on these criteria, the degree of maturation of each astrocyte can be determined.
Müller cells are radial glia in the retina. Although Müller cells help in the migration of immature neurons during retinal development, they remain in the retina after migration of ganglion cells ceases. A subpopulation of Müller cells, called peripapillary glial cells (PPGCs), are located adjacent to the optic nerve head (ONH) in the chicken retina [5-7]. PPGCs have a similar shape to Müller cells and bear bidirectional processes; the vitread processes extend toward the vitreous body, and the ventricular processes protrude toward the subretinal space. However, PPGCs are dissimilar to Müller cells with regard to antigenic phenotype [7, 8]. For example, glutamine synthetase, a specific Müller cell antigen, is not expressed in PPGCs. We recently reported that αB-crystallin, a lens protein, is exclusively expressed in PPGCs during chick retina development [8]. However, a role for PPGCs in retinal development and the phenotypic characteristics of PPGCs are still unknown. To determine if PPGCs are phenotypically similar to radial glia in the brain, we labeled PPGCs with anti-αB-crystallin antibody and tested the antigenic phenotype of these cells based on the criteria of Chan-Ling et al. [4]. We demonstrated that αB-crystallin-labeled developing PPGCs had the antigenicity of APCs, similar to radial glia in the brain; however, the antigenicity of mature PPGCs was analogous to that of mature astrocytes. We first classified the PPGC antigenic phenotypes and identified the differentiation stage of these cells.
Materials and Methods
Experimental animals
This work was approved by the institutional animal care and use committee of Chungbuk National University (approval no. CBNUA-092-0906-01). Post-hatch day 120 (P120) chickens were used in this experiment as adults. Fertilized eggs (Pulmuwon, Seoul, Korea) were incubated at 38℃ in a humid atmosphere until they reached the appropriate embryonic (E) day, according to the Hamburger and Hamilton method [9].
Tissue preparation
Eyes were quickly obtained from decapitated adult chickens and chick embryos, and the cornea, lens, and vitreous body were sequentially removed from each. Tissue was placed in 4% paraformaldehyde in phosphate buffered saline (PBS) for 3 hours. The tissue was cryoprotected by serial sucrose infiltration, embedded in OCT compound, and frozen rapidly. Tissue specimens were cut into 10-20 µm horizontal sections on a cryostat (CM 3050, Leica, Wetzlar, Germany).
Immunohistochemical staining
Retinal sections were incubated overnight at 4℃ with a monoclonal anti-αB-crystallin (1 : 500, Enzo Life Sciences International, Plymouth Meeting, PA, USA) antibody diluted in PBS. Subsequently, immunohistochemistry was performed using the avidin-biotin peroxidase complex method, as described previously [8]. After the monoclonal antibody incubation, the sections were incubated with biotinylated horse anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) diluted 1 : 400 in PBS. The sections were washed in PBS and incubated with the avidin-biotin-peroxidase complex (Vector Laboratories). Next, the sections were reacted with 0.05% 3,3'-diaminobenzidine and a 0.01% solution of hydrogen peroxide in PBS. The immunoreactions were observed under a multipurpose microscope (DMLB, Leica) with an attached digital camera system (Macrofire, Optronics, East Muskogee, OK, USA).
Double immunofluorescence
For double-immunostaining, mouse anti-αB-crystallin, mouse anti-vimentin (1 : 2, 3CB2, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), rabbit anti-Pax2 (1 : 100, Covance, Princeton, NJ, USA), and rabbit anti-GFAP (1 : 200, Dako, Glostrup, Denmark) antibodies were used as primary antibodies. For secondary antibodies, Cy2-labeled anti-rabbit IgG (1 : 300, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Cy3-labeled anti-mouse IgG (1 : 500, Jackson ImmunoResearch Laboratories) were used. Stained samples were observed under a confocal microscope (TCS SP2 AOBS, Leica).
Results
αB-crystallin expression in PPGCs from E8 to adult
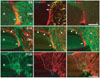
Immunostaining for αB-crystallin was performed to identify morphological changes in the αB-crystallin-positive PPGCs following maturation. At the early developmental stage (E8), αB-crystallin-immunoreactive PPGCs adjacent to the ONH had a bipolar shape with long processes traversing entire layers of the retina (arrowheads in Fig. 1A). At the late developmental stage (E18), αB-crystallin immunoreactivity disappeared in the vitread processes of PPGCs (arrows in Fig. 1B). However, the cell bodies and ventricular processes of the PPGCs contained the αB-crystallin protein (arrowheads in Fig. 1B). Additionally, αB-crystallin immunoreactivity was also observed in oligodendrocytes of the ONH and the nerve fiber layer (NFL) of the retina, similar to our previous report [8]. In the adult (P120), αB-crystallin immunoreactivity was not observed in presumptive PPGC locations (arrows in Fig. 1C). Instead, αB-crystallin-immunopositive signals were weakly observed in the ONH and the NFL of the retina.
Pax2, vimentin, and GFAP expression in αB-crystallin-positive PPGCs
Pax2, vimentin, and GFAP expression were observed in αB-crystallin-positive PPGCs to identify the astrocytic phenotypes of PPGCs. At E8 and E18, Pax2 and vimentin were expressed in αB-crystallin-positive PPGCs (Fig. 2A, B, D, E). However, GFAP immunoreactivity was not observed in PPGCs (Fig. 2C, F). Thus, developing PPGCs exhibited a Pax2-positive/vimentin-positive/GFAP-negative profile. Pax2 and vimentin were also expressed in PPGCs in the ONH at these ages. GFAP immunoreactivity was not observed in the PPGCs of the retina and the ONH at E8, suggesting that astrocyte maturation had not yet occurred in that region (Fig. 2C). In contrast to E8, GFAP was highly expressed in the ONH at E18. Interestingly, GFAP expression was not ever observed in αB-crystallin-positive PPGCs (Fig. 2F). In the adult (P120), Pax2 immunoreactivity was not observed in the presumptive location of PPGCs (data not shown). This result indicates that Pax2-expressing immature astrocytes and PPGCs lose their Pax2 antigenicity as they mature. Vimentin and GFAP were clearly observed in the presumptive location of PPGCs in the retina (dotted areas in Fig. 2G, H), as well as in the ONH. The expression of these two proteins overlapped within each location (dotted area in Fig. 2I). Mature PPGCs at P120 were Pax2-negative/vimentin-positive/GFAP-positive. Fig. 3 depicts a scheme of the astrocytic phenotype of αB-crystallin-positive PPGCs.
Discussion
αB-crystallin expression in PPGCs
Because of the absence of specific molecules for marking PPGCs, it has been difficult to distinguish these cells from surrounding structures using Golgi stains or electron microscopy. Recently, we found that αB-crystallin was exclusively expressed in PPGCs and some oligodendrocytes in the developing chick retina, and reported that αB-crystallin is a useful marker protein for observing PPGCs [8]. The results of the present study show that αB-crystallin expression in PPGCs decreased from E18 and ceased at P120. These findings raise two possibilities. One possibility is that PPGCs are transformed thoroughly into typical astrocytes, including their shape. The other possibility is that PPGC features are retained and only αB-crystallin expression decreases within PPGCs. Based on their data from previous studies, a group of researchers have reported that PPGCs have a bipolar shape with a long vitread process and a short ventricular process at early developmental stages, have lateral processes extending off of the original vitread process at middle stages, and finally have numerous fibrous lateral processes at later embryonic stages and at the adult stage. However, in their report, the PPGC processes did not shorten during maturation [6, 7, 10]. Therefore, the first possibility that PPGCs may be transformed into astrocytes like other radial glia during development is excluded. However, the reason why αB-crystallin is temporally expressed within PPGCs is currently unclear. Given the fact that αB-crystallin is a member of the small heat-shock protein family [11-13] and acts as a molecular chaperone [14, 15], it is conceivable that αB-crystallin plays a role in the survival of developing PPGCs.
Astrocytic PPGC phenotype
Pax2 is a transcription factor that plays a critical role in determining cell fate in the CNS during development [16]. Recently, it was reported that Pax2-expressing cells are immature astrocytic cells [4, 17, 18]. In the present study, developing PPGCs were Pax2-positive/vimentin-positive/GFAP-negative until hatching. Chan-Ling et al. [4] defined Pax2-positive/vimentin-positive/GFAP-negative cells phenotypically as APCs and demonstrated that APCs are highly proliferative. We injected BrdU into the yolks of E6, E14, and E18 eggs and harvested embryos 6 hours later to examine the proliferative activity of developing PPGCs. Consequently, few BrdU-labeled cells were observed in the presumptive location of PPGCs at all three stages (data not shown). Although PPGCs are phenotypically APCs, they do not show proliferative activity. These data strongly suggest that astrocytes in the ONH are not derived from PPGCs.
Several studies have indicated that astrocytes are absent in the chicken retina [8, 19]. However, a recent study reported the presence of retinal astrocytes in the chick [20]. Therefore, this issue is still controversial. Our result that Pax2-expressing astrocytes were observed in the ONH from E8 to E18, but not in the retina, strongly indicate the absence of retinal astrocytes. Indeed, Pax2 expression was not detected in the ONH or the retina at P120, suggesting that Pax2-positive immature astrocytes completely differentiated into Pax2-negative mature astrocytes in the adult eye. Additionally, Pax2-negative/vimentin-negative/GFAP-positive cells of the rodent and human retina are considered mature astrocytes [4, 21]. Mature PPGCs (Pax2-negative/vimentin-positive/GFAP-positive) also have similar antigenic phenotypes to mature astrocytes, except for vimentin. Considering that vimentin expression is prolonged until the post-hatching period in the chicken brain [22-24], it seems reasonable to conclude that mature PPGCs are phenotypically identical to mature astrocytes in this avian species.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download