Abstract
This article describes a straightforward method to measure the dimensions and identify morphological variations in the cerebral arterial circle using the general-purpose software program Osiris. This user-friendly and portable program displays, manipulates, and analyzes medical digital images, and it has the capability to determine morphometric properties of selected blood vessels (or other anatomical structures) in humans and animals. To ascertain morphometric variations in the cerebral arterial circle, 132 brains of recently deceased fetuses, infants, and adults were dissected. The dissection procedure was first digitized, and then the dimensions were measured with Osiris software. Measurements of each vessel's length and external diameters were used to identify and classify morphological variations in the cerebral arterial circle. The most commonly observed anatomical variations were uni- and bilateral hypoplasia of the posterior communicating artery. This study demonstrates that public domain software can be used to measure and classify cerebral arterial circle vessels. This method could be extended to examine other anatomical regions or to study other animals. Additionally, knowledge of variations within the circle could be applied clinically to enhance diagnostic and treatment specificity.
The cerebral arterial circle (CAC, also known as the circle of Willis [CW]) was first described in 1658 and detailed by Thomas Willis in 1664 [1-3]. The CAC is formed by three incoming arteries (the left and right internal carotid arteries and the basilar artery) and their anastomoses (the anterior and pos terior cerebral arteries). The anterior cerebral arteries are connected to each other by the anterior communicating artery (AcoA). The basilar artery bifurcates into the two posterior cerebral arteries, which are connected to the internal carotid arteries by the posterior communicating artery (PcoA) [2-4].
Many studies have been performed on the cerebral circulation due to its clinical importance and the frequency of anomalies and irregularities of the arterial circle [5-7]. A variety of techniques have been used to study variations in this vascular circle, such as angiography, magnetic resonance angiography (MRA) [5], computed tomography angiography (CTA), transcranial Doppler (TCD) [8-10], intraoperative obser vations [11], and autopsy [12]. Non-autopsy measurements have some advantages, but their shortcomings lie in the detection of anatomical variations (such as arterial duplications). They are also operator dependent; thus, interpretation of the results lacks accuracy. The main advantage of autopsy for studying the vascular circle is its direct and wide view of the vessels, which facilitates observing and measuring frequently encountered variations. With the user-friendly Osiris software, data collected from a post-mortem analysis becomes both accurate and reproducible.
This study proposes a simple method for measuring the length and external diameter of vessels forming the CAC and appropriately classifying the variations. To the best of our knowledge, using public domain software to determine the morphology of CAC vessels in a cadaver study has not been previously documented.
The present technique was carried out on 132 recently deceased Iranian fetuses (n=3), neonates (n=12), infants (n=15), and adults (n=102). Each brain was extracted from subjects who had died less than 3 hours earlier. Cases with remarkable alterations in brain arteries or evidence of gross pathological lesions (such as crush injuries, macroscopically identified cortical tumors, severe hemorrhage, or infections) of the brain were excluded. The medico-legal office and Medical Ethics Committee of the Tehran University of Medical Sciences approved this study.
The scope of this study included only the arterial circle as formed by the AcoA, the anterior cerebral artery (A1), the posterior cerebral artery (P1), and the PcoA.
After excising the brain from the cranial cavity it was placed on a flat plane, ventral surface up. Images were taken exactly perpendicular to the plane of the circle to avoid errors due to different angles of view (Fig. 1). The procedure was digitized (Canon 6.0 Megapixel digital camera, Tokyo, Japan), so as to be readily available for further studies. Each image included a metric ruler placed along the plane of the vessels at the time of photography. After downloading and installing Osiris software (Digital Imaging Unit, University Hospital of Geneva, Switzerland; http://www.sim.hcuge.ch/osiris/), opening the Osiris program allows the user to select an image to be displayed from a file location on their computer. Under the "Tools" menu on the main program console, there is a "line caliper" function that can be used to measure the length between two points on the displayed image. This tool was used to measure the various anatomical parameters and to standardize measurements using the metric ruler included in each image.
To measure 'external diameter,' we found the largest diameter of the selected vessel with the naked eye and inserted two points at the edges of the width. This created a measurable line of pixels in the Osiris software. The count of the number of pixels along the line was translated to units of length using the metric ruler (Fig. 2A). If vessels that were thrombosed or collapsed due to extravasations were found, measurements were made wherever the most realistic external diameter of the vessels occurred, which occasionally had to be estimated. The diameter of the whole fenestrated vessel was measured, as in the case of a fenestrated AcoA.
For measuring the 'length,' vessel segments were considered where they formed part of the CAC, i.e., P1 between the PcoA and the tip of the basilar artery, and A1 from its junction with the PcoA and the internal carotid artery to the point it connected to the AcoA. We first delineated a virtual crosssection where the selected vessel emerged from the proximal artery and found the same cross-section where it inserted into the distal artery. Connecting the midpoints of these cross-sections along the midline of the artery allowed the most accurate length measurement (Fig. 2B). The length of a broken line fitted on the artery was measured when the vessel had a spiral or curved course (Fig. 2C). The Osiris software was used to measure the length in the same manner as the external diameter.
To be consistent with previous work, hypoplastic vessels were defined as those with external diameters of less than half the average in fetuses and infants [13] and <1 mm in adults [14]. Asymmetry was defined as a vessel diameter that was less than half of the contralateral vessel diameter. No corrections were made for measurements of those vessels that were under mild traction due to lack of sufficient elasticity.
This method was useful to accurately identify and classify anatomical variations in the CAC. The most often observed variations in our subjects were uni- and bilateral hypoplasia of the PcoA. Thirteen fetuses and infants (44%) and 27 adults (27%) exhibited this variation in one PcoA; two fetuses and two infants (8%) and 34 adults (33%) exhibited this trait bilaterally. Hypoplasia was also commonly observed in the AcoA of adults (11%) and fetal/infant specimens (7%). Other hypoplastic vessels included the A1 and P1 segments in a unilateral fashion. Bilateral hypoplasia was only observed in PcoA vessels. Aplastic vessels were somewhat less common. Aplasia was observed in the unilateral PcoA (7%), bilateral PcoA (3%), and in one AcoA (1.0%) in adult specimens. Aplasia in the fetal/infant population was only observed in the unilateral PcoA (20%) and bilateral PcoA (3%). No aplasia of the A1 and P1 was found in any specimen. Details of the dimensions of the respective arterial segments of these 132 subjects are shown in Table 1 [1, 2].
Anatomical variations in the vascular supply to different organs are common and can be deceptive at times. The CAC is an arterial network that creates collateral circulation between the anterior and posterior and the left and right circulations of the brain. The calibers of the different segments of the CAC vary so much that no two circles are identical [3]. As discussed in previous investigations [15-17], studying this circle is important for determining the probable correlation between anatomical variations and the risk of cerebrovascular diseases. It is generally accepted that anatomical study of the CW is of great clinical and prognostic value, given the prevalence of pathologies such as atherosclerotic occlusion and aneurysms [5, 6]. The relationship between cerebral aneurysms and variations in the CAC has already been verified [16]. Some variations in the CW seem to be deadly risk factors for acute ischemic stroke [18] or related to a neurological disease such as migraine [19]. Consequent changes in the diameter of some arterial segments have been observed in the case of ischemic cerebral accidents. Such a condition may alter vascular functionality, as demonstrated by angiography and observed during autopsy [3].
Congenital variations in CAC anatomy are common. Significant variability may exist among different ethnic groups [7, 15]; however, this was not confirmed by Eftekhar et al. [2]. These variations may complicate neurosurgical interventions. Thus, it is important to determine the adequacy of the neurovasculature for providing blood to the brain during procedures for cerebral aneurysms or ligations of the carotid artery. The development of bypassing or shunting effects, vascular inadequacy, or a lack of recovery after vascular occlusion might be explained, in part, by these variations in the CW anatomy [20].
Different techniques are used to measure the dimensions of CAC for particular aims. The most popular methods are MRA [5], TCD [8-10], CTA, and autopsy. Although these are highly developed, sophisticated methods [21], they have their respective limitations. Current indirect methods (MRA, TCD, and angiography) have limited resolution and sometimes makes distinguishing CAC variations difficult (for example, hypoplasia from aplasia in an arterial segment). This can affect the prevalence of an anatomically complete CAC [18, 19, 22]. Furthermore, the operator-dependent nature of these methods may detract from the exactness of the results compared to direct visualization techniques.
Variations and dimensions in the CAC have been defined and characterized as a whole or in particular segments in several autopsy studies. However, only a few studies have described the exact method of arterial measurement [1, 2, 4, 23-27]. Some of these studies have mentioned the diameter of the formative vessels, but not the technique or instrument of measurement [3, 15, 20, 28-30]. Fixation injections (latex, micropaque, or a mixed suspension) and the type of vision (by microscope or naked eye) were detailed in some publications [4, 13, 20, 24, 31]. Some authors prepared detailed drawings and provided photographs of the CW [24]. However, they did not use photographs to measure the arteries. Seydel [4] used a micrometer for measurements, whereas others used a caliper gauge [23, 25]. A comparison of these studies is not feasible because most studies do not define their criteria for different variations, such as hypoplasia, in the same format [27]. Additionally, the lack of measurement technique details in several studies prohibits a reliable comparison.
Osiris was designed at the University Hospital of Geneva in 1990 as an extensible and portable program for displaying, manipulating, and analyzing digital images. It can be customized (without loss of basic consistency) for different clinical implementations. This software manipulates images for analysis by utilizing different tool functions, such as rotating, flipping, zooming, panning, and magnifying. Additionally, special Osiris measurement tools allow for an evaluation of distances and angles that can be used for research purposes or as a clinical tool. Images can be used for morphometric measurement studies with high analytical consistency by showing pixel intensities along a line drawn across the image [35].
The Osiris package is a non-commercial product (www.sim.hcuge.ch/osiris/). It is readily accessible to physicians and non-computer-oriented users and is easily adaptable to a physician's specific needs [35]. Our study represents the first documentation of Osiris used to measure CW arteries.
This study may solve some of the pitfalls of previous anatomical studies measuring anatomical dimensions. This technique is a simple way of obtaining measurements and variations in the CAC. It provides researchers and clinicians the ability to share digital images online for consultation with other experts and colleagues. Furthermore, using this method can save time, manpower, and money compared to radiological techniques or traditional autopsy methods.
The measurements can be repeated at any time or by any person for a comparison with other studies and to verify the results. We have used the absolute values of the measurements for morphology classifications. This minimizes the extent of error and makes the method more applicable and transferable.
Osiris software has been utilized in different fields of medicine [36-38]. Dulguerov et al. [37] used the software to mea sure movements during facial expressions recorded by a digital video camera. Magnetic resonance images have also been analyzed using Osiris in a study of dural sac movements [38] and erosion volumes in patients with rheumatoid arthritis [36]. The software has been used for the first time to measure the dimensions of the CAC and to classify anatomical variations [1, 2]. This software is not restricted to use in the CAC area, and we suggest that it can be used in other regions of the body (in humans or animals) for the purpose of measuring and classifying anatomical variations. Any software program must be easy to use if scientists are to accept it, and Osiris fulfilled its purpose in an exceedingly convenient manner.
There are some disadvantages in the described method that are shared by all CW morphological studies. First, there is a potential minor change in vessel diameter over time. Therefore, the diameter of hypoplastic vessels may increase with time and exceed 1 mm. Second, all measurements were performed postmortem. As our results were not controlled with angiography, we could never be sure that the diameters of collapsed vessels measured with Osiris software reflected their actual size in living tissue. Vessels may also be different from those measured after formalin fixation or injection techniques. This problem may raise some concerns about the reliability of our results. Finally, the number of autopsies was relatively low (132 cadavers). As other measurement sources were not available (such as cerebral angiograms during lifetime) and we could not find any published data that had used such a method of measurement in the medical literature, we cannot comment on the extent of errors. Thus, further studies with a larger number of subjects using this technique and a comparison to other methods are needed to verify these findings.
Taken together, we demonstrated the successful use of public domain software to describe dimensions and morphologic variants in the entire CAC. The simple method we used is suitable for basic comparisons of diameters and lengths or studying gross anatomical variations. Other anatomical structures could feasibly be applied to this morphometric process, and it could be equally valid for studies of animal specimens. Similar to all autopsy and radiological techniques, this method is limited in its ability to represent the dynamic nature of the CAC during life. Advances in imaging analysis may resolve this limitation and allow exceedingly accurate conclusions about CAC morphology.
Figures and Tables
Fig. 1
Photography method. (A) Schematic pictures showing the ruler at the level of the Willis circle on the frontal lobe and (B) digital picture taken perpendicular to that plane.
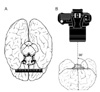
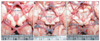
Fig. 2
The external diameter and length of the vessels were measured using Osiris software. (A) Measuring the external diameter: a/b=10/100=0.1 cm. (B) Measuring the length: a/b=122/100=1.22 cm. (C) Measuring the curved length: (a+b)/c=(55+70)/100=1.25 cm.
Acknowledgements
The authors wish to thank Drs. Branislav Vidic and Suzan Parhizgar from Texas Tech University Health Sciences Center, and David Peace from University of Florida for their invaluable help with this article.
References
1. Ardakani SK, Dadmehr M, Nejat F, Ansari S, Eftekhar B, Tajik P, El Khashab M, Yazdani S, Ghodsi M, Mahjoub F, Monajemzadeh M, Nazparvar B, Abdi-Rad A. The cerebral arterial circle (circulus arteriosus cerebri): an anatomical study in fetus and infant samples. Pediatr Neurosurg. 2008. 44:388–392.
2. Eftekhar B, Dadmehr M, Ansari S, Ghodsi M, Nazparvar B, Ketabchi E. Are the distributions of variations of circle of Willis different in different populations? Results of an anatomical study and review of literature. BMC Neurol. 2006. 6:22.
3. Lazorthes G, Gouaze A, Santini JJ, Salamon G. Le cercle artériel du cerveau (circulus arteriosus cerebri). Anat Clin. 1979. 1:241–257.
4. Seydel HG. The diameters of the cerebral arteries of the human fetus. Anat Rec. 1964. 150:79–88.
5. Macchi C, Catini C, Federico C, Gulisano M, Pacini P, Cecchi F, Corcos L, Brizzi E. Magnetic resonance angiographic evaluation of circulus arteriosus cerebri (circle of Willis): a morphologic study in 100 human healthy subjects. Ital J Anat Embryol. 1996. 101:115–123.
6. Bhagwati SN, Deshpande HG. Study of circle of Willis in 1021 consecutive autopsies: incidence of aneurysms, anatomical variations and atherosclerosis. Ann Acad Med Singapore. 1993. 22:3 Suppl. 443–446.
7. Aydın ME, Kaya AH, Kopuz C, Demir MT, Corumlu U, Dagcinar A. Bilateral origin of superior cerebellar arteries from the posterior cerebral arteries, and clues to its embryologic basis. Anat Cell Biol. 2011. 44:164–167.
8. Mitchell DG, Merton DA, Mirsky PJ, Needleman L. Circle of Willis in newborns: color Doppler imaging of 53 healthy full-term infants. Radiology. 1989. 172:201–205.
9. Macchi C, Giannelli F, Cecchi F, Gulisano M, Pacini P, Corcos L, Catini C, Brizzi E. The inner diameter of human intracranial vertebral artery by color Doppler method. Ital J Anat Embryol. 1996. 101:81–87.
10. Macchi C, Catini C, Giannelli F, Cecchi F, Gulisano M, Pacini P, Brizzi E. The blood-flow velocity analysis as a possible method for arterial calibre discovery. A statistical investigation in 108 human subjects by color-Doppler method. Ital J Anat Embryol. 1996. 101:45–55.
11. Stojanović N, Stefanović I, Randjelović S, Mitić R, Bosnjaković P, Stojanov D. Presence of anatomical variations of the circle of Willis in patients undergoing surgical treatment for ruptured intracranial aneurysms. Vojnosanit Pregl. 2009. 66:711–717.
12. Rhoton AL Jr. The supratentorial arteries. Neurosurgery. 2002. 51:4 Suppl. S53–S120.
13. Milenković Z, Vucetić R, Puzić M. Asymmetry and anomalies of the circle of Willis in fetal brain. Microsurgical study and functional remarks. Surg Neurol. 1985. 24:563–570.
14. Saeki N, Rhoton AL Jr. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg. 1977. 46:563–578.
15. Kayembe KN, Sasahara M, Hazama F. Cerebral aneurysms and variations in the circle of Willis. Stroke. 1984. 15:846–850.
16. Horikoshi T, Akiyama I, Yamagata Z, Sugita M, Nukui H. Magnetic resonance angiographic evidence of sex-linked variations in the circle of Willis and the occurrence of cerebral aneurysms. J Neurosurg. 2002. 96:697–703.
17. Hoksbergen AW, Majoie CB, Hulsmans FJ, Legemate DA. Assessment of the collateral function of the circle of Willis: three-dimensional time-of-flight MR angiography compared with transcranial color-coded duplex sonography. AJNR Am J Neuroradiol. 2003. 24:456–462.
18. Chuang YM, Liu CY, Pan PJ, Lin CP. Posterior communicating artery hypoplasia as a risk factor for acute ischemic stroke in the absence of carotid artery occlusion. J Clin Neurosci. 2008. 15:1376–1381.
19. Bugnicourt JM, Garcia PY, Peltier J, Bonnaire B, Picard C, Godefroy O. Incomplete posterior circle of willis: a risk factor for migraine? Headache. 2009. 49:879–886.
20. Alpers BJ, Berry RG, Paddison RM. Anatomical studies of the circle of Willis in normal brain. AMA Arch Neurol Psychiatry. 1959. 81:409–418.
21. Stock KW, Wetzel S, Kirsch E, Bongartz G, Steinbrich W, Radue EW. Anatomic evaluation of the circle of Willis: MR angiography versus intraarterial digital subtraction angiography. AJNR Am J Neuroradiol. 1996. 17:1495–1499.
22. Malamateniou C, Adams ME, Srinivasan L, Allsop JM, Counsell SJ, Cowan FM, Hajnal JV, Rutherford MA. The anatomic variations of the circle of Willis in preterm-at-term and termborn infants: an MR angiography study at 3T. AJNR Am J Neuroradiol. 2009. 30:1955–1962.
23. Papantchev V, Hristov S, Todorova D, Naydenov E, Paloff A, Nikolov D, Tschirkov A, Ovtscharoff W. Some variations of the circle of Willis, important for cerebral protection in aortic surgery: a study in Eastern Europeans. Eur J Cardiothorac Surg. 2007. 31:982–989.
24. Van Overbeeke JJ, Hillen B, Tulleken CA. A comparative study of the circle of Willis in fetal and adult life. The configuration of the posterior bifurcation of the posterior communicating artery. J Anat. 1991. 176:45–54.
25. el Khamlichi A, Azouzi M, Bellakhdar F, Ouhcein A, Lahlaidi A. Anatomic configuration of the circle of Willis in the adult studied by injection technics. Apropos of 100 brains. Neurochirurgie. 1985. 31:287–293.
26. Baptista AG. Studies on the arteries of the brain. II. The anterior cerebral artery: some anatomic features and their clinical implications. Neurology. 1963. 13:825–835.
27. Hillen B. The variability of the circulus arteriosus (Willisii): order or anarchy? Acta Anat (Basel). 1987. 129:74–80.
28. Fisher CM. The circle of Willis: anatomical variations. Vasc Dis. 1965. 2:99–105.
29. Riggs HE, Rupp C. Variation in form of circle of Willis. The relation of the variations to collateral circulation: anatomic analysis. Arch Neurol. 1963. 8:8–14.
30. Orlandini GE, Ruggiero C, Orlandini SZ, Gulisano M. Blood vessel size of circulus arteriosus cerebri (circle of Willis): a statistical research on 100 human subjects. Acta Anat (Basel). 1985. 123:72–76.
31. Caruso G, Vincentelli F, Rabehanta P, Giudicelli G, Grisoli F. Anomalies of the P1 segment of the posterior cerebral artery: early bifurcation or duplication, fenestration, common trunk with the superior cerebellar artery. Acta Neurochir (Wien). 1991. 109:66–71.
32. Gielecki J, Zurada A, Kozłowska H, Nowak D, Loukas M. Morphometric and volumetric analysis of the middle cerebral artery in human fetuses. Acta Neurobiol Exp (Wars). 2009. 69:129–137.
33. Gielecki JS, Syc B, Wilk R, Musiał-Kopiejka M, Piwowarczyk-Nowak A. Quantitative evaluation of aortic arch development using digital-image analysis. Ann Anat. 2006. 188:19–23.
34. Gościcka D, Gielecki J, Materka A. Application of digital image analysis system for measurements of the blood vessels parameters (diameter, length, volume). Folia Morphol (Warsz). 1990. 49:1–7.
35. Ligier Y, Ratib O, Logean M, Girard C. Osiris: a medical image-manipulation system. MD Comput. 1994. 11:212–218.
36. Bird P, Lassere M, Shnier R, Edmonds J. Computerized measurement of magnetic resonance imaging erosion volumes in patients with rheumatoid arthritis: a comparison with existing magnetic resonance imaging scoring systems and standard clinical outcome measures. Arthritis Rheum. 2003. 48:614–624.
37. Dulguerov P, Wang D, Perneger TV, Marchal F, Lehmann W. Videomimicography: the standards of normal revised. Arch Otolaryngol Head Neck Surg. 2003. 129:960–965.
38. Hirasawa Y, Bashir WA, Smith FW, Magnusson ML, Pope MH, Takahashi K. Postural changes of the dural sac in the lumbar spines of asymptomatic individuals using positional stand-up magnetic resonance imaging. Spine (Phila Pa 1976). 2007. 32:E136–E140.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download