Abstract
A low serum level of vitamin D has been associated with an increased incidence of gastrointestinal tract cancers. However, the effects of vitamin D3 have not been investigated in gastric cancer and cholangiocarcinoma. In the present study, we found that vitamin D3 treatment significantly suppressed the viability of gastric cancer and cholangiocarcinoma cells. Moreover, vitamin D3 had a synergistic effect with other anti-cancer drugs, such as paclitaxel, adriamycin, and vinblastine, for suppressing cell viability. To determine the underlying mechanism involved in the regulation of viability by vitamin D3, we examined the effects of vitamin D3 on expression of hedgehog signaling target genes, which has been associated with gastric cancer and cholangiocarcinoma. Vitamin D3 treatment decreased the level of mRNA expression of patched1, Gli1, cyclin D1, and Bcl2, suggesting the possibility that vitamin D3 may act through regulation of hedgehog signaling. From the above results, we conclude that vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma.
Vitamin D3 (1, 25-dihydroxyvitamin D3) has been associated with the regulation of Ca2+ and Pi transport and bone mineralization [1, 2]. This association has been supported by vitamin D3-related gene knockout studies [3-5]. However, recent studies indicate that vitamin D3 affects cell proliferation, differentiation, and gene regulation [2]. Moreover, three kinds of evidence suggest an association between vitamin D3 and carcinogenesis. The first class of evidence is derived from epidemiological studies. A low serum vitamin D3 level has been associated with an increased risk for colorectal [6], breast [7], and prostate cancers [8]. Moreover, Giovannucci et al. [9] studied the relationship among dietary and supplementary vitamin D, physical activity, body mass index, and sunlight exposure in 47,800 individuals and found a strong association between low levels of vitamin D3 and increased cancer incidence, particularly for cancers of the digestive system. The second class of evidence is derived from altered expression of vitamin D3-related genes in many tumor types. Overexpression of CYP24, which downregulates the level of the active form of vitamin D3, has been reported in various cancers, including colon [10, 11] and esophageal [12] cancers. Amplification of 20q13.2, which harbors the CYP24 gene, has been reported in gastric and breast cancers [13, 14]. The third class of evidence is derived from studies about vitamin D3-related gene knockout mice. In vitamin D receptor knockout mice, a higher incidence of lymphoblastic and thymic lymphoma and mammary hyperplasia is observed [15].
Gastric cancer is the second leading cause of cancer-related deaths in the world [16]. Gastric cancer is a malignant tumor that originates from gastric mucosa and is classified into intestinal and diffuse types. Many factors are related to the cause of gastric cancer, such as a high-salt diet, ingestion of grilled meats and fish, and infection by Helicobacter pylori [17]. These bacterial, environmental, and host factors contribute to the molecular changes of gastric cancer. Despite advances in diagnostic technology such as endoscopy, the cure rate for gastric cancer is still poor due to fast growth and metastasis. Cholangiocarcinoma is a malignant tumor that originates from bile duct epithelial cells [18]. Intrahepatic cholangiocarcinoma is the second most common subtype of primary hepatobilliary cancer [18, 19]. Chronic inflammation of the liver contributes to the malignant transformation of cholangiocytes. The main reasons for the poor prognosis of patients with cholangiocarcinoma are due to diagnostic difficulty, extensive local tumor invasion, and multidrug resistance. Many molecular factors contribute to carcinogenesis of gastric cancer and cholangiocarcinoma [20-23]. Interestingly, hedgehog (Hh) signaling contributes to the progression of both cancers [24].
Although vitamin D3 regulates the growth of various cancers, vitamin D3 has not been examined in gastric cancer and cholangiocarcinoma. Herein, we report that vitamin D3 inhibits cell viability in gastric cancer and cholangiocarcinoma cells.
SNU1, SNU638, and SNU1079 cells were cultured with RPMI-1640, 25 mM HEPES, 10% fetal bovine serum (FBS), and 1× penicillin/streptomycin. HuCCT1 cells were cultured in RPMI-1640, 10% FBS, and 1× penicillin/streptomycin at 37℃ in a 5% CO2 incubator. SNU1, SNU638, and SNU1079 cells were purchased from the Korea Cell Line Bank (Seoul, Korea). HuCCT1 cells were purchased from the Health Science Research Resources Bank (Osaka, Japan). Vitamin D3, cyclopamine, paclitaxel, adriamycin, and vinblastine were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cells were seeded at a density of 1×103 (SNU1 and SNU638), 1.5×103 (HuCCT1), or 4×103 cells (SNU1079) per well in 96-well plates and treated with the indicated concentrations of vitamin D3. Four days following treatment, 10 µl of Ez-Cytox reagent (ITSBio, Seoul, Korea) was added to the wells, and the cells were incubated for an additional 2 hours under normal cell culture conditions. Cell viability was measured by absorbance at 450 nm using an ELISA reader (Tecan, Mannedorf, Switzerland).
Total RNA was extracted using a RNeasy Mini kit (Qiagen, Valencia, CA, USA) and processed based on the manufacturer's manual. cDNA was synthesized with MMLV reverse transcriptase (Promega, Madison, WI, USA), dNTP, and oligo-dT primers. Real-time PCR was conducted using FastStart Universal SYBR Green Master Mix (Roche Applied Science, Indianapolis, IN, USA) in an ABI Prism 7500 sequence detector (Applied Biosystems, Foster City, CA, USA). The primer sequences were as follows: Ptch1 (F: 5'-AAC CCC TGG ACG GCC GGG AT-3', R: 5'-AGG ATG ACC ACG GGC ACG GCA-3'); Gli1 (F: 5'-TGC TGG ATG GGC GGG AGG ACC-3', R: 5'-CCC CGT GGA TGT GCT CGC TGT-3'); CCND1 (F: 5'-CGC GCA GAC CTT CGT TGC CCT-3', R: 5'-GCC TTG CAC TGC GGC CAC CA-3'); Bcl2 (F: 5'-CTG GGG GAG GAT TGT GGC CTT CTT TG-3', R: 5'-TCC AGG TGT GCA GGT GCC GGT TC-3'); and β-actin (F: 5'-GCG AGC ACA GAG CCT CGC CT-3', R: 5'-GCC TTG CAC ATG CCG GAG CC-3').
All data are presented as mean±SD. All experiments were repeated at least four times. The difference between the mean values of the two groups was evaluated using the Student's t-test (unpaired comparison). We used a one-way analysis of variance, followed by Tukey's multiple comparison to compare more than three groups. A P-value<0.05 was considered statistically significant.
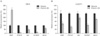
We used SNU1 and SNU638 cells to test the effect of vitamin D3 on the viability of gastric cancer cells. Cells were seeded in 96-well plates and treated with different concentrations of vitamin D3 in the presence of 1% FBS. After 4 days, we measured the absorbance. Vitamin D3 treatment significantly inhibited the viability of gastric cancer cells compared to that in control cells in a dose-dependent manner (Fig. 1A, B). Specifically, treatment with 10 µM vitamin D3 suppressed viability by >80%. Subsequently, we tested the effect in HuCCT1 and SNU1079 (cholangiocarcinoma) cells. Vitamin D3 treatment inhibited viability of cholangiocarcinoma cells as in gastric cancer cells (Fig. 1C, D).
Brüggemann et al. [25] reported that vitamin D3 does not exert a synergistic effect with other anti-cancer drugs. However, it remains unclear whether or not vitamin D3 has a synergistic effect with other anti-cancer drugs in gastric cancer and cholangiocarcinoma cells. Thus, we seeded SNU1 and HuCCT1 cells in 96-well plates and added vitamin D3 with anti-cancer drugs, such as paclitaxel, adriamycin, and vinblastine in the presence of 10% FBS. Cell viability was measured after 2 days of treatment. As shown in Fig. 2, a significant difference was observed in cell viability between the vitamin D3 alone and the combined with anti-cancer drugs groups. Treatment with vitamin D3 alone decreased the average survival rate of SNU1 and HuCCT1 cells by 57% and 41% respectively (Fig. 2). However, combined treatment with other drugs decreased the survival rate by >70%. This result indicates that vitamin D3 can function synergistically with other anti-cancer drugs.
To investigate the underlying mechanism involved in the regulation of viability by vitamin D3, we examined Hh signaling because cyclopamine, a specific inhibitor of the Hh signaling pathway, almost completely suppressed cancer cell viability (Fig. 1). Furthermore, Bijlsma et al. [26] reported that vitamin D3 is an antagonist of Hh signaling. However, the relationship between vitamin D3 and Hh signaling is not well-studied in cancer cells. To determine whether or not vitamin D3 is associated with Hh signaling in cancer cells, we assessed Hh signaling activity by measuring the Ptch1 and Gli1 expression levels, which are direct target genes of Hh signaling. SNU1 and HuCCT1 cells were treated with 10 µM vitamin D3 one day after cell seeding. One day following the treatment, the Ptch1 and Gli1 mRNA expression level decreased sharply (Fig. 3). We also examined effects on the expression of other Hh signaling targets, including cyclin D1 and bcl2. In particular, we have shown previously that inhibiting Hh signaling reduces bcl2 expression in gastric cancer cells [27]. Vitamin D3 treatment reduced cyclin D1 and bcl2 expression (Fig. 3). These results suggest that vitamin D3 may act as an antagonist of Hh signaling in gastric cancer and cholangiocarcinoma cells.
Vitamin D3 has been widely used clinically, and the proper dosage and side effects have been well-documented [28]. In the present study, we showed, for the first time, that gastric cancer and cholangiocarcinoma cells are sensitive to vitamin D3, and that vitamin D3 has synergism with other cancer drugs. Moreover, we also showed that the actions of vitamin D3 are possibly mediated through inhibition of Hh signaling.
The expression of Hh signaling target genes was regulated by vitamin D in the present study (Fig. 3). Hh signaling is one of the most important signaling pathways regulating proliferation, differentiation, embryogenesis, and cancer [29]. In mammals, Hh consists of three different members, including Sonic hedgehog, Indian hedgehog, and Desert hedgehog [30]. The trigger for signaling is the Hh ligand binding to a 12-pass transmembrane protein patched (Ptch1), which resembles the Niemann-Pick disease type C1 (NPC1) protein. The NPC1 protein is involved in cholesterol trafficking and has a pump function [26]. After Hh binds to Ptch1, Ptch1 no longer blocks the 7-pass transmembrane protein, smoothened (Smo), and subsequently the intracellular signaling cascade is activated, including Fused, protein kinase A, GSK3, CKI, and Gli. Finally, the Gli protein is activated and translocated to the nucleus where it turns on target genes. However, in the absence of Hh, Smo is continuously repressed by Ptch1. Finally, the Hh target genes are turned off.
The association between vitamin D and Hh signaling was first reported by Bijlsma et al. [26]. Bijlsma et al. [26] reported that Ptch1 can secrete 3β-hydroxysteroids, which inhibit Smo on other cells. Vitamin D3 directly binds to Smo with high affinity in a cyclopamine-sensitive manner. Moreover, Bijlsma et al. [26] showed that treating zebrafish embryos with vitamin D3 mimics the Smo-/- phenotype. Their report suggested that vitamin D3 can be used as an anti-cancer drug. In contrast, Brüggemann et al. [25] reported that vitamin D3 inhibits pancreatic cancer cell viability in the presence of 0.5% serum; however, it does not inhibit the pancreatic cancer cell viability in the presence of 10% serum. Moreover, vitamin D3 did not show any synergism with other anti-cancer drugs for inhibiting cell viability. Brüggemann et al. [25] suggested that vitamin D3 is not an efficient anti-cancer drug. However, in the present study, vitamin D3 had the ability to inhibit cell viability and showed a synergistic effect with other anti-cancer drugs in 10% serum, as shown in Fig. 2. Hence, our data indicate that vitamin D3 could be used as an anti-cancer drug, and its effect may depend on cell type.
Vitamin D3 is related to cancer via various signaling pathways [2]. However, in the present study, we focused on Hh signaling because cyclopamine, a specific inhibitor of Hh signaling, almost completely suppressed cancer cell viability, which was consistent with previous reports [24]. Although it is unclear how the Hh signaling pathway is activated in gastric cancer and cholangiocarcinoma, it is clear that Hh signaling activity is critical for viability [24, 27]. We showed that vitamin D3 reduced the expression of Hh signaling target genes (Fig. 3), suggesting the possibility that Hh signaling may play a critical role in vitamin D3-induced inhibition of viability. Future studies such as Gli1 overexpression are required to confirm this possibility.
Figures and Tables
Fig. 1
Effect of vitamin D3 on gastric cancer cell and cholangiocarcinoma cell viability. SNU1 (A), SNU638 (B), HuCCT1 (C), and SNU1079 (D) cells were treated with the indicated concentrations of vitamin D3 in 96-well plates in the presence of 1% fetal bovine serum. The cells were further incubated for 4 days, and the viability assay was performed. Ethanol (vehicle) or cyclopamine (10 µM) were used as negative and positive controls, respectively. Data are expressed as percent change (mean±SD) compared to the control (*P<0.01, ANOVA followed by Tukey's multiple comparison).

Fig. 2
Synergistic effect of vitamin D3 with anti-cancer drugs. SNU1 (A) and HuCCT1 (B) cells were treated with paclitaxel (0.5 nM), adriamycin (ADR, 150 nM), or vinblastine (100 nM) with or without vitamin D3 (35 µM) in the presence of 10% fetal bovine serum. The cell viability assay was performed 2 days after treatment. Ethanol (vehicle) was used as the negative control. Data are expressed as percent change (mean±SD) compared to the control (*P<0.01, compared to the single treatment, Student's t-test).

Fig. 3
Vitamin D3 regulates the expression of Hh signaling target genes. SNU1 (A) and HuCCT1 (B) cells were seeded at 1×105/well in 6-well plates. After 1 day, the cells were treated with 10 µM vitamin D3. One day later, RNA was purified from the cells, and real-time PCR was performed. Ethanol (vehicle) was used as the negative control. Data are expressed as percent change (mean±SD) compared to control (*P<0.01, †P<0.05, Student's t-test).
References
1. Holick MF. Vitamin D and bone health. J Nutr. 1996. 126:4 Suppl. 1159S–1164S.
2. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007. 7:684–700.
3. Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001. 98:7498–7503.
4. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997. 16:391–396.
5. Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997. 94:9831–9835.
6. Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989. 2:1176–1178.
7. Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005. 14:1991–1997.
8. Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000. 11:847–852.
9. Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006. 98:451–459.
10. Cross HS, Bises G, Lechner D, Manhardt T, Kállay E. The Vitamin D endocrine system of the guts: its possible role in colorectal cancer prevention. J Steroid Biochem Mol Biol. 2005. 97:121–128.
11. Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006. 57:234–240.
12. Mimori K, Tanaka Y, Yoshinaga K, Masuda T, Yamashita K, Okamoto M, Inoue H, Mori M. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann Oncol. 2004. 15:236–241.
13. Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet. 2000. 25:144–146.
14. Weiss MM, Snijders AM, Kuipers EJ, Ylstra B, Pinkel D, Meuwissen SG, van Diest PJ, Albertson DG, Meijer GA. Determination of amplicon boundaries at 20q13.2 in tissue samples of human gastric adenocarcinomas by high-resolution microarray comparative genomic hybridization. J Pathol. 2003. 200:320–326.
15. Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005. 97:153–164.
16. Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003. 3:592–600.
17. Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006. 12:2979–2990.
18. Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004. 66:167–179.
19. Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004. 40:472–477.
20. Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004. (157):327–349.
21. Wise C, Pilanthananond M, Perry BF, Alpini G, McNeal M, Glaser SS. Mechanisms of biliary carcinogenesis and growth. World J Gastroenterol. 2008. 14:2986–2989.
22. Francis H, Alpini G, DeMorrow S. Recent advances in the regulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2010. 299:G1–G9.
23. Rashid A. Cellular and molecular biology of biliary tract cancers. Surg Oncol Clin N Am. 2002. 11:995–1009.
24. Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003. 425:846–851.
25. Brüggemann LW, Queiroz KC, Zamani K, van Straaten A, Spek CA, Bijlsma MF. Assessing the efficacy of the hedgehog pathway inhibitor vitamin D3 in a murine xenograft model for pancreatic cancer. Cancer Biol Ther. 2010. 10:79–88.
26. Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006. 4:e232.
27. Han ME, Lee YS, Baek SY, Kim BS, Kim JB, Oh SO. Hedgehog signaling regulates the survival of gastric cancer cells by regulating the expression of Bcl-2. Int J Mol Sci. 2009. 10:3033–3043.
28. Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007. 85:6–18.
29. Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008. 15:801–812.
30. Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993. 75:1417–1430.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download