Abstract
Renal epithelial cells damaged by ischemia/reperfusion (I/R) can be restored by timely and appropriate treatment. Recent studies have reported that intra renal adult kidney stem cells contribute to the restoration of tubules damaged by I/R. Here, we determined the role of adult tubular cells in the restoration of damaged tubules. We labeled slow cell-cycle cells (SCCs) with 5-bromo-2'-deoxyuridine (BrdU) and investigated their location in the kidneys as well as their contribution to the restoration of tubular cells damaged by I/R injury in mice. Thirty minutes of bilateral ischemia resulted in severe disruption of tubular epithelial cells along with a decline in renal function. The post-ischemic disruption of tubular epithelial cells was most severe in the S3 segment of the outer stripe of the outer medulla. Damaged tubules demonstrated gradual recovery of renal function over time. BrdU-labeled SCCs were mainly observed in tubules located at the junction of cortex and outer medulla, as well as in the inner medulla. The tubular SCCs expressed functional tubule cell markers such as Na/K-ATPase, Na-K-Cl cotransporter-2, and aquaporin 1 and 2. BrdU-labeled SCCs survived I/R injury and proliferated. These results demonstrate that SCCs present in tubules contribute to the restoration of tubular epithelial cells injured by I/R.
Ischemia/reperfusion (I/R) in the kidney induces damage to kidney cells, including tubular epithelial cells and endothelial cells, resulting in renal functional disorder. I/R in the kidney is one of the most common causes of acute kidney injury (AKI) [1, 2]. AKI is a common complication in hospitalized patients, particularly in medical and surgical intensive care units [2]. The mortality rate in hospitalized AKI patient ranges from 30 to 80% [2, 3]. Despite therapeutic advances, mortality has remained high over several decades.
Renal tubular epithelial cells injured by I/R insult are restored after appropriate reflow of blood. It has been recognized that restoration of damaged renal tubular epithelial cells accounts for the differentiation and/or proliferation of mildly damaged but surviving intra-renal cells [4, 5]. Recently, some evidence has demonstrated that extra-renal cells such as bone marrow-derived stem cells (BMDC) contribute to the restoration of damaged tubules by incorporation into the damaged tubules [6-9]. These findings suggest that cells originated from outside of the kidney can be used in cell-based treatment for AKI. However, accumulating evidence including our results has demonstrated that bone marrow-derived mesenchymal stem cells are not a major contributor to the restoration of damaged tubular epithelial cells after I/R injury, although BMDCs improve renal function after I/R injury [4, 10, 11]. Recently, the presence of adult stem cells in the kidney as well as their contribution to the restoration of tubules injured by I/R were reported [12-15]. However, the kinetic changes and distribution of intra-renal adult stem cells during tubular recovery from I/R injury have not been clearly defined yet.
In the present study, we investigated the location and role of 5-bromo-2'-deoxyuridine (BrdU)-labeled slow cell-cycle cells (SCCs), which are recognized as adult stem cells, in the kidney as well as their role in the restoration of tubules injured by I/R in mice. Here, we observed that the label-retaining SCCs were located mainly in the tubular epithelia in the deep cortex and papilla, and injured tubules were restored when these cells divided. These results indicate that the kidney possesses intra-renal stem cells and that intra-renal stem cells contribute to the restoration of damaged tubules, suggesting that timely replenishment of damaged tubular epithelial cells by the activation of intra-renal cell proliferation can be an important therapeutic strategy for the treatment of AKI.
Experiments were performed using 8-week-old BALB/c male mice (Koatech, Namyangju, Korea). All experiments were carried out according to animal experimental procedures approved by the Animal Care and Use Committee of Kyungpook National University. Each animal group consisted of at least four mice. Mice were anesthetized with pentobarbital sodium (60 mg/kg body weight; intraperitoneally), and kidneys were exposed through flank incision. Kidneys were subjected to ischemia by clamping renal pedicles with non-traumatic microaneurism clamps (Roboz, Rockville, MD, USA). After 30 minutes, the clamps were removed and blood reflow conformed. Body temperature was maintained at 36.6-37.5℃ throughout the procedure.
Newborn mice were administrated with 50 mg/kg body weight of BrdU (Sigma-Aldrich, St. Louis, MO, USA) intraperitoneally from 12 hours after birth for 6 days.
Seventy microliters of blood was taken from the retrobulbar vein plexus at the times indicated in the figures. Plasma creatinine (Pcr) concentrations were measured using a Beckman Creatinine Analyzer II (Beckman, Brea, CA, USA).
Kidneys were perfused via the left ventricle with phosphate buffered saline (PBS) for 2 minutes at 37℃ and then with 4% PLP (4% paraformaldehyde, 75 mM L-lysine, 10 mM sodium periodate) fixative. Kidneys were excised, placed in PLP overnight, washed, and stored in PBS containing 0.02% sodium azide at 4℃ until use. Fixed tissue was embedded in paraffin and then cut into 4 µm sections using a microtome. Kidney sections were stained with periodic acid Schiff stain.
Kidney sections were deparaffinized with xylene and rehydrated with serial dipping in 100, 95, and 80% ethanol. To unmask the epitopes of antigens, the kidney sections were boiled in 10 mM sodium citrate buffer, pH 6.0, for 15 minutes. To block the activation of endogenous peroxidase, the kidney sections were treated with 3% H2O2 in methanol for 30 minutes at 4℃. Kidney sections were blocked with blocking buffer containing 2% normal horse serum for F6, 2% bovine serum albumin for BrdU, and 2% normal goat serum for aquaporin (AQP)1 and AQP2 staining for 30 minutes at room temperature. The sections were incubated in primary antibodies (BrdU [Sigma-Aldrich], Na/K-ATPase [F6, Sigma-Aldrich], AQP1 and AQP2 [Alomone Lab. Jerusalem, Israel], and Na-K-Cl cotransporter-2 [NKCC2; Alpha Diagnostic International, San Antonio, TX, USA]) appropriately diluted with respective blocking buffers overnight at 4℃. The sections were then washed with PBS three times and incubated with biotinylated respective secondary antibodies for 60 minutes at room temperature. Sections were treated with ABC reagent (Vector, Burlingame, CA, USA) and substrate, then washed with tap water. Sections were mounted with a permount solution and observed by a light microscope (Nikon Instrument Inc., Tokyo, Japan).
To investigate the histological damage of tubular epithelial cells after I/R injury, we carried out a kinetic morphological analysis at 1, 2, 3, 4, 5, 7, 9, and 16 days after 30 minutes of bilateral renal ischemia in mice. Thirty minutes of bilateral renal ischemia and subsequent reperfusion resulted in the disruption and congestion of tubules (Fig. 1). The loss of tubular epithelial cells and tubular congestion were most severe in the outer stripe of the outer medulla in mice (Fig. 1). The loss of tubular epithelial cells in the outer stripe of the outer medulla peaked at 2 days after I/R and decreased over time (Fig. 1, arrows indicate damaged tubules), indicating that damaged tubules were restored overtime. In the cortex and inner medulla, loss of tubular epithelial cells and congestion was mild compared with that in the outer stripe of outer medulla (Fig. 1).
Consistent with the level of kidney tubular epithelial cell damage, 30 minutes of bilateral ischemia resulted in an increase in PCr (Fig. 2A). The increase in PCr peaked at 2 days after I/R, then gradually returned to a normal level overtime (Fig. 2A). Body weights were negatively correlated with PCr concentrations and degrees of histological tubular epithelial cell damage (Fig. 2B).
Since adult stem cells have slow cell-cycle properties [12, 16], we investigated the presence of SCCs in adult kidneys as well as their contribution to the restoration of disrupted kidney tubular epithelial cells after I/R. To locate the SCCs, mice were administered thymidine analogue BrdU (50 mg/kg body weight, ip) for 6 days beginning at 12 hours after birth. After a chase phase of 8 weeks, mice were subjected to 30 minutes of bilateral renal ischemia. Eight weeks after BrdU-administration, BrdU-positive SCCs were located sporadically throughout the kidneys but prominently on the tubules of the deep cortex and papilla (Fig. 3A, B). BrdU-positive cells on the tubules expressed typical membrane transporters according to the tubule segments; Na/K-ATPase in the proximal tubules, AQP2 in the collecting ducts, and NKCC2 in the distal tubules (Fig. 3C). This indicates the presence of SCCs in physiologically differentiated adult renal tubules.
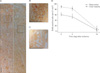
To evaluate the contribution of BrdU-retaining SCCs to the restoration of damaged tubular epithelial cells, we exposed BrdU-incorporated mice to 30 minutes of bilateral ischemia and evaluated the distribution and number of BrdU-retaining cells in the kidney. Since BrdU-signals of BrdU-retaining cells fade out in response to both cell death and division, we first determined the numbers and distribution of BrdU-retaining cells 5 days after ischemia, which is the end-phase of cell death and early-phase of recovery of damaged tubules after I/R. Many BrdU-positive cells were detected on the tubules of the deep cortex and papilla 5 days after ischemia (Fig. 4A, B), indicating that the BrdU-retaining tubular cells survived I/R injury. Sixteen days after ischemia, BrdU-retaining cells were not easily detected in the kidney, and the number of BrdU-positive cells was significantly reduced when compared with that 5 days after ischemia (Fig. 4A, B). This indicates that BrdU-retaining cells that survived I/R injury contributed to the restoration of damaged tubules presumably by proliferation.
In the present study, we report that label-retaining SCCs, which mainly reside on the deep cortex and papilla, contribute to the restoration of damaged kidney tubules after I/R injury. As it is known that the outer stripe of the outer medulla is most susceptible to I/R injury [2, 17-19], most of the tubules in the outer stripe of the outer medulla were disrupted 24 to 72 hours following 30 minutes of ischemia in mice. The number of damaged tubules gradually decreased with time along with functional recovery. This suggests that the damaged tubular epithelial cells were restored and differentiated to mature functional tubular epithelial cells.
After I/R, most of the tubular epithelial cells in the S3 segment of the proximal tubules are disrupted and removed from the tubules [17]. Meanwhile, some of the damaged but surviving tubules are known to undergo recovery immediately to several days after I/R [5, 10, 17]. This suggests that surviving cells within or adjacent to damaged tubules may be primary sources of regenerated cells [20-22]. Kim et al. [13, 14] reported that adult renal stem cells reside predominantly in the S3 segment of the proximal tubules in humans, which suggests that the mitotic division of resident stem cells might be stimulated by cellular injury. In a recent study, we found that tubular epithelial cells in the deep cortex proliferate very quickly and intensively after I/R injury [17]. Besides, several other studies have demonstrated that cells in the corticomedullary junction contribute to the restoration of damaged tubular epithelial cells [14, 17, 20]. In the present study, we found that label-retaining SCCs in the deep cortex that survived I/R injury disappeared simultaneously with the restoration of damaged tubules. The disappearance of label-retaining cells means that label-retaining cells divided and proliferated, contributing to the restoration of damaged tubule cells. It is possible that the fade-out of BrdU signaling was due to the death of BrdU-labeled cells. However, cell death can not a major reason for the loss of BrdU-labeled tubular cells since the renal function and morphology were much improved. In addition, BrdU-labeled cells in the renal papilla, which were less susceptible to I/R, also faded out. Therefore, we speculate that the reduction of BrdU-positive cells was mainly caused by cell division. In an uranyl acetate-induced acute renal failure model of rat, which causes focal proximal tubule depletion in the S3 segment, it has been suggested that the restoration of damaged tubular epithelial cells occurs by SCCs in the S3 segment [23-25].
Oliver et al. [15] reported that the inner medulla is a niche for stem cells that contribute to the restoration of post-ischemic damaged tubular epithelial cells. In the present study, we found label-retaining cells also in the inner medulla following I/R, and the label-retaining cells that survived I/R disappeared in the recovery phase. This suggests that the label-retaining cells in the inner medulla also contributed to the regeneration of tubular cells. On the other hand, the papilla is far away from the outer stripe of the outer medulla (several millimeter distance between the outer stripe of the outer medulla and the inner medulla in adult mice). Therefore, in the restoration of the S3 segment of proximal tubules, the label-retaining cells in the papilla might not be a major contributor in the early phase of recovery. Probably, the proliferation of label-retaining cells in the papilla could contribute to the restoration of tubules located in the papilla or tubules in the outer medulla during late phase.
In the present study, we found that BrdU-positive cells were the majority of cells in the cortico-medullar region, which is just above the outer stripe of the outer medulla, and possessed a membrane transporter, indicating maturity, suggesting that vigorous proliferation of SCCs started later then 5 days after I/R. In a previous study, we found that after I/R injury, cell proliferation started at about post-ischemic day 3, peaked at day 4, and maintained a high level until day 9. These results suggest that, although both label-retaining and non-label cells contributed to restoration of damaged tubular epithelia, cell proliferation occurred at a very early point after ischemia, and cell restoration may be governed mainly by non-label retaining cells. Maeshima et al. [26] performed BrdU-injection for 7 days followed by 2 weeks of chase phase in adult rat and reported that 18 hours after ischemia, BrdU-labeled cells expressed vimentin, which disappeared over time into gradual expression of E-cadherin, which is an epithelial marker. In that study, they suggested that in early phases of tubular regeneration, descendents of label-retaining cells dedifferentiated first, followed by maturation into tubular epithelial cells later [26]. However, to clarify the kinetic roles of label-retaining and non-label-retaining cells, further studies are needed.
In conclusion, our present study demonstrates that intra renal resident cells have stem- or progenitor-like characteristics and contribute to the restoration of damaged tubular epithelial cells, suggesting that timely activation of mitosis by intra renal stem cells can be an important therapeutic strategy for AKI treatment.
Figures and Tables
Fig. 1
Post-ischemic tubular cell damage. BALB/c male mice were subjected to 30 min of bilateral renal ischemia. Kidneys were harvested at the indicated times and periodic acid Schiff-stained. Severe tubular epithelial cell damage was revealed in the outer stripe of the outer medulla in the kidneys. Arrows indicate damaged tubules. Scale bar=250 µm.
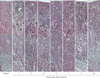
Fig. 2
Plasma creatinine (A) and body weight (B) after 30 min of bilateral ischemia. Male BALB/c mice were subjected to either 30 min of ischemia or sham-operation and plasma creatinine, and body weights were determined at 1, 2, 3, 4, 5, 7, 9, and 16 days after the operation. Values presented are expressed as the mean±SEM (n=5).

Fig. 3
Numbers and characteristics of BrdU-retaining cells in the kidneys. Twelve hours after birth, mice were administered with 50 mg/kg body weight of BrdU, ip, for 6 days. After a chase period of 8 wk, mouse kidneys were harvested. (A) Kidney sections were immunostained using anti-BrdU antibody, and images were taken at lower magnification. The right column is a high magnification image. (B) BrdU-positive cells were counted in 0.1-mm2 fields of the superficial cortex, deep cortex, outer medulla, and inner medulla of the kidney (10 fields per kidney). Results are expressed as the mean±SE (n=4). (C) Kidney sections were double-stained with anti-BrdU, -Na/K-ATPase, -Na-K-Cl cotransporter-2 (NKCC2), -aquaporin (AQP)1, or -AQP2 antibody. Images were obtained in the corticomedullary junctions. Arrows indicate BrdU-positive cells. Scale bar=250 µm (A), 50 µm (C).
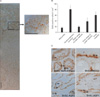
Fig. 4
Change in BrdU-retaining cell number after ischemia/reperfusion injury. Twelve hours after birth, mice were administered with 50 mg/kg body weight of BrdU, ip, for 6 days. Eight weeks after the chase period, mouse kidneys were subjected to 30 min of bilateral ischemia and harvested 5 and 16 days after ischemia. (A-C) Kidney sections were immunostained using anti-BrdU antibody, and images were taken at low (A) and high (B, C) magnifications. (B and C) are high magnification images of the left and right dot boxes of (A), respectively. Scale bars=250 µm. (D) BrdU-positive cells were counted. Results are expressed as the mean±SE (n=4). *P<0.05 vs. the deep cortex and inner medulla of day 0 in Fig. 3, †P<0.05 vs. the inner medulla at respective time point.
Acknowledgements
This work was supported by the Korea Research Foundation KRF-2005-202-E00015 (to K.M.P.).
References
1. Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005. 68:1613–1617.
2. Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996. 334:1448–1460.
3. Brodie JC, Humes HD. Stem cell approaches for the treatment of renal failure. Pharmacol Rev. 2005. 57:299–313.
4. Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005. 115:1743–1755.
5. Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994. 93:2175–2188.
6. Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002. 62:1285–1290.
7. Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003. 112:42–49.
8. Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003. 14:1188–1199.
9. Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001. 195:229–235.
10. Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003. 14:Suppl 1. S55–S61.
11. Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005. 115:1756–1764.
12. Kim J, Park KM. Locations of slow-cycling cells, adult stem cells, in the organs of adult mouse. Korean J Anat. 2007. 40:347–357.
13. Kim K, Lee KM, Han DJ, Yu E, Cho YM. Adult stem cell-like tubular cells reside in the corticomedullary junction of the kidney. Int J Clin Exp Pathol. 2008. 1:232–241.
14. Kim K, Park BH, Ihm H, Kim KM, Jeong J, Chang JW, Cho YM. Expression of stem cell marker CD133 in fetal and adult human kidneys and pauci-immune crescentic glomerulonephritis. Histol Histopathol. 2011. 26:223–232.
15. Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004. 114:795–804.
16. Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000. 97:13473–13475.
17. Kim J, Kim JI, Kwon TH, Park KM. Kidney tubular cell regeneration starts in the deep cortex after ischemia. Korean J Nephrol. 2008. 27:536–544.
18. Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem. 2001. 276:11870–11876.
19. Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002. 277:2040–2049.
20. Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005. 166:545–555.
21. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008. 2:284–291.
22. Loverre A, Capobianco C, Ditonno P, Battaglia M, Grandaliano G, Schena FP. Increase of proliferating renal progenitor cells in acute tubular necrosis underlying delayed graft function. Transplantation. 2008. 85:1112–1119.
23. Fujigaki Y, Goto T, Sakakima M, Fukasawa H, Miyaji T, Yamamoto T, Hishida A. Kinetics and characterization of initially regenerating proximal tubules in S3 segment in response to various degrees of acute tubular injury. Nephrol Dial Transplant. 2006. 21:41–50.
24. Fujigaki Y, Sakakima M, Sun Y, Fujikura T, Tsuji T, Yasuda H, Hishida A. Cell division and phenotypic regression of proximal tubular cells in response to uranyl acetate insult in rats. Nephrol Dial Transplant. 2009. 24:2686–2692.
25. Sakakima M, Fujigaki Y, Yamamoto T, Hishida A. A distinct population of tubular cells in the distal S3 segment contributes to S3 segment regeneration in rats following acute renal failure induced by uranyl acetate. Nephron Exp Nephrol. 2008. 109:e57–e70.
26. Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003. 14:3138–3146.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download