Abstract
The superior cerebellar artery is the most consistent branch of the basilar artery and arises near the bifurcation of the basilar artery. A bilateral origin of the superior cerebellar arteries from the posterior cerebral arteries has been rarely reported in the literature. Reporting variations in brain vessels is important for neurosurgeons to safely and confidently treat pathologies in this region. We report on a specimen with a bilateral origin to the superior cerebellar artery from the posterior cerebral artery and discuss the embryogenesis of this rare variation.
The anatomy of the posterior circulation is very complex and variable [1]. Even within consistent territory, different variations in the superior cerebellar artery (SCA) have been described including duplication, triplication, and extraordinary origins [2-5], whereas the bilateral occurrence of these variations have been encountered less frequently.
A bilateral origin of the SCA from the posterior cerebral artery (PCA) is very rarely reported [5, 6]. Although, there is no evidence of clinical findings related to this variation, it is important radiologically, because it displays an unusual appearance. The anatomical variations or anomalies of these vessels have recently become significant because of imaging techniques such as computed tomography and magnetic resonance imaging.
The existence of the such anatomical variations and vascular pathology can also be explained by the embryological development in this region. Moreover, the developmental basis of this variation is worthy of discussion in light of the literature. Therefore, we report a case that had bilateral origins of the SCAs from the PCAs.
The variation was encountered during a preliminary stage of a study on the cerebral arterial territory in 20 cadaveric brain specimens taken from adults aged 20 to 60 years. The cadavers were embalmed with a mixture of 10% formalin, 50% methanol, 10% glycerin, and 30% water. The supraclavicular and carotid regions, internal carotid artery (ICA), and the vertebral artery of specimens were filled with red latex injected prior to dissection. The distal parts of both arteries were ligated, and the brains were subsequently removed carefully. Microsurgical dissection was performed on arteries of the Circle of Willis. We conducted a complete evaluation of the Circle of Willis in all brains. The vascular anatomy of colored vessels was studied using a surgical loop under magnification (×3) and digital photographs were taken of the vascular structures after latex injection and dissection.
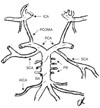
In our case, the SCAs bilaterally arose from the PCAs. The right and left SCAs originated from the posterolateral aspect of the PCAs at points 4 and 4.5 mm proximal to the rostral ends of the basilar arteries (BAs) and the origins of the PCAs, respectively (Figs. 1, 2). The arteries took a lateral course and branched at the surface and lateral face of the cerebellum (rostral=medial and caudal=lateral trunks). The SCAs were separated from the PCAs by the oculomotor nerve. The outer diameter of the SCAs at their origins was 3 mm. The outer diameters of the right and left PCAs and BA were 5, 5.5, and 6.5 mm, respectively. The BA origin was at the pontomedullary sulcus. The SCA bifurcation into the rostral (medial) and caudal (lateral) trunks occurred at a distance 25 mm on the right side and 32 mm on the left side from the SCA origin. The anterior inferior cerebellar arteries originated from the BA at a point 16 mm distal from the rostral end of the BA. The diameter of the anterior inferior cerebellar artery was 1.5 mm on both sides. The other brain vessels had no anomalies in their courses or in the origin of their branches on either side (Figs. 1, 2).
Many authors have described the anatomy of the posterior circulation of the brain and its variations. Pai et al. [1] described variation in the SCA with different origin points as a single trunk and rarely with a double trunk and most commonly at the P1 segment of the PCA and P1 segment, which is also called the precommunicating segment that extends from the basillar bifurcation to the junction of the PCA with the posterior communicating artery (PCOMA).
The SCA is the most consistent artery in the posterior circulation in terms of origin and location [1, 7]. The BA bifurcation is an important determinant of the initial course of the SCA [1, 8].
A normal PCA arises at the bifurcation of the BA, is joined by the PCOMA, and is divided into four segments (P1-P4) throughout its course. In one case, the left SCA originated from the P1 segment of the PCA and sometimes rarely the SCA arose as a double trunk from the BA [1]. In normal vascular anatomy, the SCA divides into two trunks as the rostral and caudal trunk. The rostral (medial) trunk supplies the vermis and the paravermian area. The caudal (lateral) trunk supplies the hemispheres on the suboccipital surface [1, 9]. Pai et al. [1] found that the SCA arose from the P1 segment in one case. Embryologically, the SCA arises from the ICA. The P1 segment of the PCA usually enlarges during development to form a major connection between the BA and PCA with a subsequent reduction in size of the PCOMA [1]. Anomalies in the P1 segment are rarely found and this may cause some identification problems during surgical dissections of this region. Furthermore, the SCA may be difficult to recognize during surgery, as only the presence of the third cranial nerve identifies it [6].
SCAs are mentioned as most consistent arterial territory of the infratentorial group and usually originate bilaterally from the distal portion of the BA as a single main trunk [3, 8, 10, 11].
In the classical description, the main stem of the SCA arises from the BA and courses along the pontomesencephalic fissure below the occulomotor nerve, then curves posteriorly through the cerebellomesencephalic fissure up to the anterior edge of the tentorial surface [8]. During this course, the SCA bifurcates mostly at the lateral pontomesencephalic level, and different names have been used for these branches such as rostral and caudal [8], superior and inferior [12], and lateral and medial branches [10]. The rostral trunk tends to supply the vermis with adjacent cerebellar hemispheric portions, whereas the caudal trunk tends to supply the more lateral cerebellar hemisphere, such as the superior part of the petrosal surface.
The SCA branches include the perforans, which is direct or indirect in type and usually originates more proximally to supply the brainstem and precerebellar branches, which supply the deep cerebellar parts. The cortical branches supply the tentorial, upper petrosal, and superior suboccipital surfaces of the cerebellum, and the cortical branches, in turn, give rise to hemispheric and vermian branches more distally [8, 10-12].
Different variations of the SCA including duplication, triplication, and an origin with the PCA have been reported previously. Duplication of the SCA was found in 28% [13], 14% [4], 20% [3], 5.9% [5], and 25% [11] of cases. Triplication was also noted in 2% [4] and 8% [2] of cases in some studies. Bilateral duplication is another variation that is very rare and considered to occur at a rate of 2% [4, 5]. Origin of the SCA from the PCA was also considered and found in 4% [4] and 2.6% of cases [5], but a bilateral occurrence of this variation is extremely rare [5, 6]. In their previous study, Dağcınar et al. [11] did not encounter another abnormal SCA origin except the presented case.
A brief review of the embryogenesis of the cerebral circulation may be helpful to understand this rare variation, namely the bilateral origin of the SCAs from the PCAs. In 4-mm embryos, the carotid system supplies the forebrain and contributes to perfusing the hindbrain via primitive segmental arteries [14, 15]. The vertebral arteries develop from a pair of primitive longitudinal arteries connected laterally to the primitive hindbrain plexus [13, 16, 17]. The ICA forms an intracranial connection with the rostral end of the longitudinal neural arteries, the PCOMA [9, 15].
In 31-day embryos, all blood supply for the cerebrum comes from the ICA (stage 2). The development of the primitive form of the PCA occurs at stage 3 (33-day-old embryos). The vertebral arteries begin to form and continued with BA formation of the posterior circulation. If full development is not completed, variations will occur, which will play an important role in the presence of aneurysmal vascular pathology [6, 15].
During the eighth gestational week, when the forebrain expands posterior, the PCA develops at the caudal end of the PCOMA as a posterior continuation of the PCOMA and the ICA. Communication to the BA develops later. In 5-8-mm embryos, the BA forms by fusion with the longitudinal neural arteries [9, 13, 16, 17].
We propose the following ontogenetic interpretations for this anomaly: 1) Lack of normal fusion of the BA at the origin of the SCA during development of the BA from the primitive neural arteries. 2) The PCA, which connects to the carotid system and primitive neural arteries, anastomose with the BA caudally at point lower than the normal site.
Thus, the origin of the SCA from the PCA is most probably associated with the unusual development of the distal basillary region during embryogenesis.
The microsurgical anatomy of the posterior circulation has many variable vascular structures. Surgery poses more risks and complications in this region, because of the presence of important vascular and neural structures. Thus, a descriptive knowledge of the anatomy of this area with its variable vascular structures will be useful for clinicians at any stage of surgical planning.
Figures and Tables
Fig. 1
Posterior circulation system is partly seen on a basal view of the injected brain. Note that the SCAs originates bilaterally from the PCAs rather than from the distal BA. AICA, anterior inferior cerebellar artery; BA, basilar artery; ICA, internal carotid artery; ON, oculomotor nerve; PB, pontine branches; PCA, posterior cerebral artery; PCOMA, posterior communicating artery; SCA, superior cerebellar artery.

Fig. 2
Schematic diagram of Fig. 1. AICA, anterior inferior cerebellar artery; BA, basilar artery; ICA, internal carotid artery; PB, pontine branches; PCA, posterior cerebral artery; PCOMA, posterior communicating artery; SCA, superior cerebellar artery.
References
1. Pai BS, Varma RG, Kulkarni RN, Nirmala S, Manjunath LC, Rakshith S. Microsurgical anatomy of the posterior circulation. Neurol India. 2007. 55:31–41.
2. Mani RL, Newton TH, Glickman MG. The superior cerebellar artery: an anatomic-roentgenographic correlation. Radiology. 1968. 91:1102–1108.
3. Salamon G, Huang YP. Radiologic anatomy of the brain. 1976. Berlin: Springer-Verlag;305–306.
4. Hardy DG, Peace DA, Rhoton AL Jr. Microsurgical anatomy of the superior cerebellar artery. Neurosurgery. 1980. 6:10–28.
5. Uchino A, Sawada A, Takase Y, Kudo S. Variations of the superior cerebellar artery: MR angiographic demonstration. Radiat Med. 2003. 21:235–238.
6. Caruso G, Vincentelli F, Rabehanta P, Giudicelli G, Grisoli F. Anomalies of the P1 segment of the posterior cerebral artery: early bifurcation or duplication, fenestration, common trunk with the superior cerebellar artery. Acta Neurochir (Wien). 1991. 109:66–71.
7. Rhoton AL Jr. Rhoton AL, editor. The cerebellar arteries. Rhoton-Cranial Anatomy and Surgical Approaches. 2003. Schaumberg: Lippincott Williams and Wilkins;461–500.
8. Rhoton AL Jr. The cerebellar arteries. Neurosurgery. 2000. 47:3 Suppl. S29–S68.
9. Lang J. Skull base and related structures: atlas of clinical anatomy. 2001. 2nd ed. New York: Schattauer Stuttgart;23–30.
10. Yaşargil MG. Microneurosurgery. 1984. New York: Georg Thieme Verlag;133–134.
11. Dagcinar A, Kaya AH, Aydın ME, Kopuz C, Senel A, Demir MT, Corumlu U, Celik F, Sam B. The superior cerebellar artery: anatomic study with review. Neurosurg Q. 2007. 17:235–240.
12. Hoffman HB, Margolis MT, Newton TH. Newton TH, Potts DG, editors. The superior cerebellar artery. Section I. Normal gross and radiographic anatomy. Radiology of the Skull and Brain: Angiography. Vol. 2. 1974. St. Louis: C.V. Mosby;1809–1830.
13. Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol. 1948. 32:205–261.
14. Chaturvedi S, Lukovits TG, Chen W, Gorelick PB. Ischemia in the territory of a hypoplastic vertebrobasilar system. Neurology. 1999. 52:980–983.
15. Cagnie B, Barbaix E, Vinck E, D'Herde K, Cambier D. A vertebral artery without atlantic and intradural sections: a case report and a review of the literature. Ann Anat. 2005. 187:271–275.
16. De Caro R, Serafini MT, Galli S, Parenti A, Guidolin D, Munari PF. Anatomy of segmental duplication in the human basilar artery: possible site of aneurysm formation. Clin Neuropathol. 1995. 14:303–309.
17. Tsutsumi M, Kazekawa K, Tanaka A, Ueno Y, Nomoto Y. Duplication of distal part of the basilar artery. Radiat Med. 2002. 20:265–267.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download