Abstract
Neuregulin-1 (NRG1) plays important roles in the development and plasticity of the brain, and has also been reported to exhibit potent neuroprotective properties. Although ErbB4, a key NRG1 receptor, is expressed in multiple regions in the adult animal brain, little is known about its role in Alzheimer's disease (AD). AD is characterized by progressive impairment of cognition and behavioral disturbance that strongly correlate with degeneration and death of neurons in the cerebral cortex and limbic brain areas, such as the hippocampus and the amygdala. Here, we show that the ErbB4 and phospho-ErbB4 immunoreactivities were higher intensity in the neurons of the CA1-2 transitional field of AD brains as compared to age-matched controls. Also, ErbB4 expression was increased in the neurons of the cortico medial nucleus amygdala, human basal forebrain and superior frontal gyrus of AD brains. In cerebral cortex and hippocampus of amyloid precursor protein/presenilin 1 double transgenic mice, ErbB4 immunoreactivity significantly increased in comparison to age-matched wild type control. These results suggest that up-regulating of ErbB4 immunoreactivity may involve in the progression of pathology of AD.
Alzheimer's disease (AD), the most common form of dementia, is a chronic neurodegenerative disease causing progressive impairment of memory and other cognitive functions. Pathologically, the AD brain is characterized by two types of lesion: senile or neuritic plaques and neurofibrillary tangles (NFT). Neuronal and synaptic loss are the essential neuropathological feature in AD, thus leading to severe impairment of neurotransmission and resulting in a decline in cognitive abilities [1]. Research on AD has been greatly stimulated by the identification of causative mutations in the genes encoding amyloid precursor protein (APP) and presenilins (PS1 and PS2). Transgenic mouse models expression APP and presenilin variants associated with familial AD have provided important insights into structural, neurophysiologic, and behavioral effects of Aβ accumulation in the brain [2].
Neuregulin-1 (NRG1) signaling participates in numerous neurodevelopmental processes, and is implicated in nerve cell differentiation and synapse formation [3, 4], radial glia formation and neuronal migration [5, 6], oligodendrocyte development and axon myelination [7, 8], axon navigation [9], and neurite outgrowth [10, 11]. NRG1 and its receptor ErbB tyrosine kinases are expressed not only in the developing nervous system, but also in adult brain. In the adult, NRG1 signaling suppresses both the induction and the expression of long-term potentiation at CA1 synapses and stimulates gamma-aminobutyric acid (GABA) release in response to depolarization. Inhibition of NRG1 signaling in hippocampal slices from postnatal mice destabilizes synaptic AMPA receptors and leads to the loss of synaptic N-methyl D-aspartate (NMDA) and spines [12]. These findings suggest additional functions of NRG1 signaling in the mature nervous system. NRG1 acts by stimulating a family of single-transmembrane receptor tyrosine kinases called ErbB [13]. NRG1 function is largely mediated by a class of receptor tyrosine kinases including ErbB2, ErbB3, and ErbB4 [14, 15]. ErbB4 is likely to be the major mediator of NRG1 functions in the brain. Especially ErbB4 is the only autonomous NRG1-specific ErbB that can both interact with the ligand and become activated by it as a tyrosine kinase. ErbB4 tyrosin kinase is expressed in multiple regions not only in the developing brain, but also in the adult brain [16, 17]. The function of NRG1 in the brain has gained much attention since the initial discovery [18] and subsequent confirmation [19] that nrg1 gene is linked to schizophrenia. Further studies showed that erbB4 gene, but not erbB2 or 3, has been shown to associate with schizophrenia [20, 21]. A subgroup of patients with late-onset AD also develops psychosis during progression of the disease (AD with psychosis). Psychotic symptoms in AD are typically defined by the presence of delusions and hallucinations [22]. A recent study suggests that NRG1 plays a role in increasing the genetic risk to positive symptoms of psychosis in a proportion of late-onset AD families [23]. The aim of our study is to further explore the involvement of ErbB4 in AD pathogenesis. We herein investigate the detailed histopathological changes in the neuron of AD brains and APP/PS1 double transgenic mice.
Antibodies were supplied by Santa Cruz Biotechnology Inc. (ErbB4, sc-283, sc-8050; p-ErbB4, sc-33040) (Santa Cruz, CA, USA) and COVANCE (beta-amyloid, SIG-39320) (Emeryville, CA, USA). Vectashield (H-1000), biotinylated anti-rabbit IgG (BA-1000) and biotinylated anti-mouse IgG (BA-2001) were supplied by Vector Laboratories (Burlingame, CA, USA). Hoechst 33342 (bis-benzimide H33342 trihydrochloride, 14533) and DAB (3,3'-diaminobenzidine tetrahydrochloride, D-5905) were supplied by Sigma Aldrich (St. Louis, MO, USA).
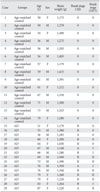
Fourteen age-matched control brains with no clinical or neuropathologic evidence of AD were obtained within 24 postmortem hours from the cadavers of the Department of Anatomy and Neuroscience, School of Medicine at Eulji University. Consent was given for brain donation by next-of-kin in all cases, and a postmortem examination was conducted by the Anatomy laboratory according to standardized protocols. All experimental procedures were performed in accordance with 'The Guidelines of the Institutional Review Board (Ethics Committee) at Eulji University.' A total of fourteen hemispheres of brains obtained by autopsy were fixed in 10% neutral buffered formalin. The fixed hemispheres were cut in coronal plane, and blocks were selected from 10 to 15 brain areas for pathologic diagnosis and a comprehensive evaluation of the neurodegenerative process. They were prepared according to routine histological procedures for paraffin section. The fifteen Alzheimer brains were generously gifted from Dr. Roger A. Brumback and were diagnosed by him (Department of Pathology, School of Medicine, Creighton University, Omaha, NE, USA). All brains were processed according to same protocols. The sections were cut in coronal plane at 8 µm for immunohistochemical study or 20 µm for confocal microscopic immunofluorescence study, and then mounted on poly-L-lysine coated slides. The sections were deparaffinized and hydrated according to routine graded xylene-alcohol methods. Hematoxylin and eosin (H&E) and Luxol fast blue/cresyl violet (LFB-CV) stains were done for general histopathology and modified silver stain by Campbell et al. [24] for detecting the senile plaque and Gallyas stain [25] for NFT were employed. The modified Campbell's silver staining method is a combination of the Campbell and Gallyas stain. The technique was modified in our laboratory for the detection of brain amyloid and NFT in humans. Briefly, deparaffinized slides are placed into a pyridine silver solution for the induction of nucleation sites, which is followed by incubation in a Gallyas' physical developer solution for 8-10 minutes. The sections are then checked under the microscope. The Gallyas developer is composed of three solutions. Solution A contains 50 g of sodium carbonate dissolved in 1,000 ml of distilled water (DW). Solution B contain 2 g of ammonium nitrate, 2 g of silver nitrate, and 10 g of tungstosilicic acid dissolved in 1,000 ml of DW. Solution C is the same as the B solution, except 7 ml of 40% formol is added. All solutions are mixed prior to the staining process (8 : 10 : 2 vol %; sol. A : sol. B : sol. C). The process is then fixed in 3% sodium thiosulfate solution in order to remove the unreacted silver and stop the silver impregnation and then rinsed in tap water for 30 minutes. The detailed data of each brain is summarized in Table 1.
The mouse model used in this study was the double transgenic APPSwe/PS1 (B6C3-Tg (APPswe, PSEN1dE9)85Dbo/J) from Jackson Laboratories (Bar Harbor, Maine). APPSwe is the Swedish mutation of the amyloid precursor protein, and PS1 is the mutant form of human presenilin 1. The sixteen-month-old wild type and transgenic mice were anesthetized with a mixture (0.01 mg/g body weight, i.p.) of ketamine (10 mg/ml) and rumpun (1 mg/ml). Mice were subjected to intracardiac perfusion-fixation using 4% paraformaldehyde dissolved in phosphate buffered saline (PBS), pH 7.4. Brains were removed and then prepared according to routine histological procedures for paraffin sectioning. The sections, cut 6 µm in thickness, were deparaffinized and hydrated according to routine graded xylene-alcohol methods. H&E stain was done for general histopathology.
In order to retrieve antigenecity, dewaxed sections were boiled within 0.1 mol/L citrate-buffered saline (pH 6.0) for 10 minutes. After cooling down for 30 minutes, the sections were rinsed in PBS. The endogenous peroxidase was quenched by 1% hydrogen peroxide in 10% methanol for 30 minutes. After two changes of PBS-T (0.1% Triton X-100 in 0.1 mol/L PBS, pH 7.6) washing for 5 minutes respectively, the sections were blocked for 1 hour in blocking solution (5% host serum+1% bovine serum albumin in PBS-T) and incubated in primary antibody (1 : 200, anti-ErbB4 and 1 : 200, APP) at 4℃ overnight. After PBS-T rinses, the sections were incubated in a biotinylated secondary antibody for 1 hour at room temperature (RT). After PBS rinsing and an avidin-biotin-peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories) treatment for 1 hour at RT, the sections were developed for 5 minutes in a 0.05% DAB solution. Images were captured with a Axiocam digital camera (MRC, Carl Zeiss Inc., Göttingen, Germany) attached on the Olympus AX70 microscope (Olympus Co., Tokyo, Japan).
Immunofluorescence was performed as previously described [26]. In brief, the prestaining process was the same as above. Normal horse serum was then applied for 1 hour to block nonspecific background staining. Then sections were incubated with avidin-biotin blocking solution (Vector Laboratories) and incubated overnight at 4℃ in blocking solution containing rabbit anti-ErbB4 (1 : 50). Following this, they were washed and incubated with the appropriate secondary biotinylated antibody and after rinsing, the sections were incubated with FITC avidin D. The sections were then incubated with Hoechst 33342 for 30 minutes and mounted. The images were visualized using the ErbB4 with FITC (green) fluorescence and Hoechst 33342 with UV (blue) fluorescence on the same sections, using a LSM 510 meta system (Zeiss LSM 510 laser scanning microscope, Carl Zeiss Inc.). To reduce auto fluorescence, the brain sections were incubated in 10% Sudan black B solution for 5 minutes, and then rinsed with disilled, deionized water (DW) and mounted with Vectashield [27].
Western blotting was carried out as previously described [17]. Briefly, cells were lysed in a modified RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 0.25% sodium-deoxycholate, 1 mM PMSF, 1 mM EDTA, 1 µg/ml aprotinin, lepeptin, and pepstain protease inhibitors). Protein was then resolved using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes and subsequently blocked with TBS containing 5% nonfat powdered milk and 0.05% Tween 20 for 1 hour. Following this, membranes were incubated overnight at 4℃ with primary antibodies and developed using horseradish peroxidase-conjugated secondary antibodies and an enhanced chemiluminescence system (Amersham Pharmacia, Buckinghamshire, UK).
Within each region from three tissue sections, two images were captured at evenly spaced intervals to represent the entire anatomic area of interest. To determine the optical density of the immunoreactive cell, we used customized Image J software (NIH). Statistical analysis was conducted with Graph Pad Prism (GraphPad software, San Diego, CA, USA). Data were presented as means±standard error of the mean of three or more independent experiments. For multiple group comparisons, statistical differences were calculated using one-way ANOVA followed by Bonferroni's test. For comparison of means from the same group of cells, a Student's paired t-test was used. Values of P<0.05 were considered significant.
All brains (control and AD) were assigned Braak scores using neuropathological staging by Braak and Braak [28]. The two braak groups are referred to as age-matched control (no pathologic sign) or AD (Braak stage I-II) referring to the extent of NFT and senile plaque in the hippocampus. Postmortem examination confirmed the diagnosis of AD given the because the presence of abundant modified Campbell silver stained amyloid deposits and NFT; H&E and LFB-CV staining also demonstrated marked cell loss (Fig. 1A, B).
Previous biochemical studies have shown that NRG1 exerts neuropreotective effects in Alzheimer's cell model via ErbB4 [29]. However, it is not clear ErbB4 immunoreactivity was changed in the neurons of AD brain. Since ErbB4 immunoreactivity is associated with neuritic plaques [30], we hypothesized that ErbB4 immunoreactivity was changed in the neuron of hippocampal fields of AD brains. As expected, ErbB4 immunoreactivity was significantly increased in the pyramidal neurons of the CA1-2 transitional field of AD brains as compared to age-matched controls (Fig. 2B, left column and 2C). As shown in Fig. 2B (right column) and 2C, phospho-ErbB4 immunoreactivity was also more increased in the same fields. At high magnification, the signal was revealed in pyramidal neurons. ErbB4 immunoreactivity was found primarily in pyramidal neurons; a subset of smaller, multipolar neurons was also observed. ErbB4 immunoreactivity was further confirmed using confocal immunofluorescence on the same section. We found that the intensity of immunoreactivity of ErbB4 was higher in the neurons of the CA1-2 transitional field of AD brains as compared to age-matched controls (Fig. 2D, E). These results are in agreement with observations above that detected with immunostaining method. This suggests that ErbB4 mediated signaling is involved in AD processing.
AD is strongly correlated with neuronal cell loss in the limbic structures, such as hippocampus and the amygdala, and the associated regions of cerebral cortex. To further determine whether ErbB4 immunoreactivity was changed in the corticomedial nucleus amygdala of AD brains. As shown in Fig. 3A and D, ErbB4 immunoreactivity was significantly increased in comparison to age-matched control. The cholinergic neurons of the human basal forebrain (BFCN) undergo selective loss in neurodegenerative disorders of the elderly, particularly AD [31]. Thus, we investigated the signals of ErbB4 in the neurons of BFCN. As shown in Fig. 3B and E, ErbB4 immunoreactivity was significantly increased in comparison to age-matched control. Unlike limbic stuructures, loss of pyramidal neurons in the laminae III and V of the superior fornatal gyrus was not severe in early AD. As AD progresses, the pyramidal neurons with apoptotic morphology (dark degeneration) appeared more in the layer V. As shown in Fig. 3C and F, ErbB4 immunoreactivity more intense in the pyramidal neuron of AD.
Double transgenic mice express a Swedish mutation (APP K595N/M596L) and a mutant human presenilin 1 (PSEN1dE9). APP/PS1 double transgenic mice develop numerous neuritic plaques throughout the hippocampus, entorhinal cortex (data not shown), and cerebral cortex (Fig. 4A). In cerebral cortex and hippocampus of APP/PS1 double transgenic mice, ErbB4 immunoreactivity significantly increased in comparison to age-matched wild type control (Fig. 4A, B). Consistent with the ErbB4 expression observed in human AD, the intensity of immunoreactivity of ErbB4 was higher in the neuron of AD. To assess whether the level of expression of ErbB4 is altered in APP/PS1 double transgenic mice, lysates were prepared from the cerebral cortex and hippocampus. A protein of the expected 185 kD size was readily detected in immunoblots with the anti-ErbB4 antibody (Fig. 4C), but there were no differences in the level of ErbB4 expression in age-matched wild type control versus APP/PS1 double transgenic mice, in agreement with earlier studies [30].
Several studies of the distribution of AD pathologic changes indicate that dementia affects predominantly the cerebral cortex; that subpopulation of neurons characterized by particular regional and laminar distributions, as well as distinct connectivity patterns, appears to be highly vulnerable [32, 33]. In addition, severe neuronal and synapse loss that mainly involves the hippocampal formation and association neocortical areas is also consistently observed in the brain of AD patients and has been shown to be a strong correlate of cognitive decline in demented people [34, 35]. Superior frontal gyrus is a part of motor association cortex which is related with judgment and planning in intellectual process. Basal forebrain cholinergic dysfunction is also a consistent feature of AD, which has been suggested to cause, at least partly, the cognitive deficits observed in patients with AD [31]. The amygdala is a gray mass situated in the dorsomedial portion of the temporal lobe. It has been known that the amygdala is involved from the early AD. The cholinergic innervations of amygdala in human is severely affected in case of AD [36].
Several previous studies have examined the localization of ErbB4. ErbB4 mRNA is enriched in regions where interneurons are clustered in rodent brains [37], and ErbB4 protein was preferentially expressed at GABAergic terminals in the prefrontal cortex [16, 17]. We previously find highly increased ErbB4 protein in the apoptotic pyramidal neurons of hippocampus of the human brain relative to pathology staging [38]. Some recent reports have shown that ErbB4 protein was expressed in the excitatory pyramidal neurons in the juvenile and adult monkeys [39]. Typically, layers III and V pyramidal neurons in cortical areas were marked by intense immunostaining in the adult human brain [40]. It appears that there are apparent species differences in the cerebral distribution of ErbB4. More work is needed to determine the neuronal subtypes that contain ErbB4.
In this study, we examined the ErbB4 immunoreactivity in the predominantly vulnerable areas of AD. The major findings of this study are as follows. First, ErbB4 and phospho-ErbB4 immunoreactivities were significantly increased in the neurons of the CA1-2 transitional field of AD brains. Second, ErbB4 expression was increased in the neurons of the corticomedial nucleus of the amygdala, BFCN, and the superior frontal gyrus of AD brains.
As in the human brain, in the cerebral cortex and the hippocampus of APP/PS1 double transgenic mice, ErbB4 immunoreactivity was significantly increased in comparison to age-matched wild type controls. In the brain, the pyramidal cell layer was strongly labeled in a dense plexus of GABAergic terminals and the boutons encircled the pyramidal cell bodies [41]. Interestingly, our study shows similar ErbB4 expression surrounding the soma of pyramidal-shaped neuron. This study provides evidence that ErbB4 may be present at GABAergic terminals. In this study, we found that increased ErbB4 immunoreactivity localized and surrounded the soma of pyramidal-shaped neurons. However, the identification of the neuronal subtype that expresses ErbB4 requires further investigation.
ErbB4 expression is widespread in various parts of the brain and nervous system [16, 17]. Recently, ErbB4 has been shown to play a key role in activity-dependent maturation and plasticity of excitatory synaptic function [12]. Further, NRG1 has been demonstrated to activate ErbB4 and regulate GABAergic transmission in adult brains [17]. Moreover, it was shown that NRG1 can be neuroprotective for cortical neurons [42], motor neurons [43], dopaminergic neurons [44], cochlear sensory neurons [45] and PC12 cells [29, 46]. It has also exhibited neuroprotection following ischemia [47-49]. These findings suggest that NRG1/ErbB4 signaling might be important in cognition, learning and memory formation through the modulation of synaptic plasticity and neuronal survival and is, therefore, a critical molecule in neurodegenerative disease. More work is needed to determine whether increased ErbB4 immunoreactivity is involved in neuroprotection or not.
Similar to APP, ErbB4 is a substrate for γ-secretase and as such, represents the first cleavage by TNF-alpha converting enzyme (TACE) to release a soluble extracellular peptide that contains the NRG1 binding site (ecto-ErbB4). The remaining membrane-anchored 80 kDa fragment (that is, ErbB4-CTF) is further cleaved in its transmembrane domain by presenilin-dependent γ-secretase to release the ErbB4 intracellular domain (ErbB4-ICD), which has been shown to translocate to the nucleus and to regulate transcription [50, 51]. Recent studies have provided that β-site APP-cleaving enzyme 1 (BACE1) participates in the proteolytic processing of NRG1 [52, 53] and involved in NRG1/ErbB4 signaling [54]. Although, more studies is needed to determine whether BACE1-dependent NRG1 processing or γ-secretase-dependent ErbB4 processing have a physiological function in central nervous system, it is possible that the balance between the generation of the APP intracellular domain and NRG1 or ErbB4-ICD are associated with brain disease including AD. The results of the current study suggest that NRG1/ErbB4 signaling may be involved in AD neuropathology. However, a more detailed analysis is needed to determine whether alterations of ErbB4 expression are important event in AD pathology. Further investigation of ErbB4 signaling could be useful in understanding the pathogenesis of AD.
Figures and Tables
Fig. 1
Detailed analysis of pathological changes in Alzheimer's disease (AD) brains. (A) Histological analysis of semi-serial paraffin sections from AD hippocamal formation. Hematoxylin and eosin staining (H&E), Luxol fast blue/cresyl violet (LFB-CV) staining, and modified Campbell's silver staining is sequentially applied to the same sections, and the same fields at CA1. (B) Quantitative analysis of data in (A) that is neuronal loss of the hippocampal pyramidal neurons. Scale bars=100 µm. Shown are means±standard errors of the mean; n=14 for age-matched control and n=15 for AD. *P<0.05.

Fig. 2
Expression of ErbB4 and p-ErbB4 in CA1-2 of hippocampus of Alzheimer's disease (AD) brains. Paired low-power composites (A) and high-power (B) images of ErbB4 (left column) and p-ErbB4 (right column) immunoreactivity in a single hippocampal tissue section. Inset, enlarged areas. (C) Quantitative analysis of data in (B) that is ErbB4 and p-ErbB4 expressing neurons. (D) ErbB4 confocal immunoflorescence staining. Nuclei are stained with Hoechst 33342. (E) Quantitative analysis of data in (D) that are ErbB4 expressing neurons. Arrowheads, pyramidal neurons; IHC, immunohistochemistry. Scale bars=100 µm (A, B, D), 25 µm (inset [enlarged areas] of B). Shown are means±standard errors of the mean; n=14 for age-matched control and n=15 for AD. *P<0.05, **P<0.01.
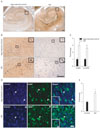
Fig. 3
ErbB4 immunohistochemistry (IHC) in the corticomedial nucleus of amygdala, cholinergic neurons of the human basal forebrain (BFCN), and superior frontal gyrus of Alzheimer's disease (AD) brains. (A, D) Hematoxylin and eosin (H&E), Luxol fast blue/cresyl violet (LFB-CV) and modified Campbell's silver staining and ErbB4 immunoreactivity in the corticomedial nucleus of amygdala. (B, E) H&E, LFB-CV and modified Campbell's silver staining and ErbB4 immunoreactivity in the BFCN. (C, F) H&E, LFB-CV and modified Campbell's silver staining and ErbB4 immunoreactivity in the superior frontal gyrus. (D-F) Quantitative analysis of data in (A-C) that are ErbB4 expressing neurons. Scale bars=100 µm, 25 µm (inset [enlarged areas]). Shown are means±standard errors of the mean; n=14 for age-matched control and n=15 for AD. *P<0.05, **P<0.01.

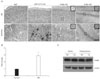
Fig. 4
ErbB4 immunohistochemistry (IHC) in presenilin/amyloid precursor protein (PS/APP) mice. (A) Hematoxylin and eosin (H&E) and Aβ (6E10), ErbB4 immunoreactivity in the cortex and hippocampus. (B) Quantitative analysis of data in (A) that are ErbB4 expressing neurons. Scale bars=100 µm, 10 µm (inset [enlarged areas]). Shown are means±SEM; n=5 for age-matched control and n=5 for PS/APP mice. *P<0.01. (C) Immunoblot analysis of ErbB4 expression in cerebral cortex and hippocampus from PS/APP mice. AD, Alzheimer's disease; Wt, wild type; Tg, transgenic.
Acknowledgements
This study was supported by grants from the National Research Foundation of Korea (NRF-2009-0074146 and NRF-2010-0005971). We thank Sangmee Ahn for providing the APP/PS1 double transgenic mice.
References
1. Tilley L, Morgan K, Kalsheker N. Genetic risk factors in Alzheimer's disease. Mol Pathol. 1998. 51:293–304.
2. Morrissette DA, Parachikova A, Green KN, LaFerla FM. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem. 2009. 284:6033–6037.
3. Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001. 11:287–296.
4. Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004. 7:575–580.
5. Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997. 124:3501–3510.
6. Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997. 19:39–50.
7. Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron. 2000. 28:81–90.
8. Calaora V, Rogister B, Bismuth K, Murray K, Brandt H, Leprince P, Marchionni M, Dubois-Dalcq M. Neuregulin signaling regulates neural precursor growth and the generation of oligodendrocytes in vitro. J Neurosci. 2001. 21:4740–4751.
9. López-Bendito G, Cautinat A, Sánchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marín O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006. 125:127–142.
10. Bermingham-McDonogh O, McCabe KL, Reh TA. Effects of GGF/neuregulins on neuronal survival and neurite outgrowth correlate with erbB2/neu expression in developing rat retina. Development. 1996. 122:1427–1438.
11. Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004. 27:379–393.
12. Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007. 54:583–597.
13. Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007. 19:124–134.
14. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001. 2:127–137.
15. Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003. 284:14–30.
16. Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003. 13:252–264.
17. Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, Lai C, Xiong WC, Gao TM, Mei L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007. 54:599–610.
18. Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002. 71:877–892.
19. Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006. 60:132–140.
20. Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006. 103:6747–6752.
21. Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006. 141B:142–148.
22. DeMichele-Sweet MA, Sweet RA. Genetics of psychosis in Alzheimer's disease: a review. J Alzheimers Dis. 2010. 19:761–780.
23. Go RC, Perry RT, Wiener H, Bassett SS, Blacker D, Devlin B, Sweet RA. Neuregulin-1 polymorphism in late onset Alzheimer's disease families with psychoses. Am J Med Genet B Neuropsychiatr Genet. 2005. 139B:28–32.
24. Campbell SK, Switzer RC, Martin TL. Alzheimers paques and tangles: a controlled and enhanced silver staining method. Abstr Soc Neurosci. 1987. 13:678.
25. Gallyas F. Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung. 1971. 19:1–8.
26. Murata K, Dalakas MC. Expression of the costimulatory molecule BB-1, the ligands CTLA-4 and CD28, and their mRNA in inflammatory myopathies. Am J Pathol. 1999. 155:453–460.
27. Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999. 47:719–730.
28. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991. 82:239–259.
29. Di Segni A, Shaharabani E, Stein R, Pinkas-Kramarski R. Neuregulins rescue PC12-ErbB-4 cells from cell death induced by beta-amyloid peptide: involvement of PI3K and PKC. J Mol Neurosci. 2005. 26:57–69.
30. Chaudhury AR, Gerecke KM, Wyss JM, Morgan DG, Gordon MN, Carroll SL. Neuregulin-1 and erbB4 immunoreactivity is associated with neuritic plaques in Alzheimer disease brain and in a transgenic model of Alzheimer disease. J Neuropathol Exp Neurol. 2003. 62:42–54.
31. Wu CK, Thal L, Pizzo D, Hansen L, Masliah E, Geula C. Apoptotic signals within the basal forebrain cholinergic neurons in Alzheimer's disease. Exp Neurol. 2005. 195:484–496.
32. Morrison JH. Differential vulnerability, connectivity, and cell typology. Neurobiol Aging. 1993. 14:51–54.
33. Giannakopoulos P, Hof PR, Bouras C. Selective vulnerability of neocortical association areas in Alzheimer's disease. Microsc Res Tech. 1998. 43:16–23.
34. Gómez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996. 16:4491–4500.
35. Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997. 278:412–419.
36. Emre M, Heckers S, Mash DC, Geula C, Mesulam MM. Cholinergic innervation of the amygdaloid complex in the human brain and its alterations in old age and Alzheimer's disease. J Comp Neurol. 1993. 336:117–134.
37. Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991. 6:691–704.
38. Woo RS, Lee JH, Yu HN, Song DY, Baik TK. Expression of ErbB4 in the apoptotic neurons of Alzheimer's disease brain. Anat Cell Biol. 2010. 43:332–339.
39. Thompson M, Lauderdale S, Webster MJ, Chong VZ, McClintock B, Saunders R, Weickert CS. Widespread expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain. Brain Res. 2007. 1139:95–109.
40. Bernstein HG, Lendeckel U, Bertram I, Bukowska A, Kanakis D, Dobrowolny H, Stauch R, Krell D, Mawrin C, Budinger E, Keilhoff G, Bogerts B. Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull. 2006. 69:546–559.
41. Wyeth MS, Zhang N, Mody I, Houser CR. Selective reduction of cholecystokinin-positive basket cell innervation in a model of temporal lobe epilepsy. J Neurosci. 2010. 30:8993–9006.
42. Li BS, Ma W, Jaffe H, Zheng Y, Takahashi S, Zhang L, Kulkarni AB, Pant HC. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J Biol Chem. 2003. 278:35702–35709.
43. Ricart K, Pearson RJ Jr, Viera L, Cassina P, Kamaid A, Carroll SL, Estévez AG. Interactions between beta-neuregulin and neurotrophins in motor neuron apoptosis. J Neurochem. 2006. 97:222–233.
44. Zhang L, Fletcher-Turner A, Marchionni MA, Apparsundaram S, Lundgren KH, Yurek DM, Seroogy KB. Neurotrophic and neuroprotective effects of the neuregulin glial growth factor-2 on dopaminergic neurons in rat primary midbrain cultures. J Neurochem. 2004. 91:1358–1368.
45. Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004. 24:8651–8661.
46. Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2) O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001. 276:46379–46385.
47. Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging. 2004. 25:935–944.
48. Li Y, Xu Z, Ford GD, Croslan DR, Cairobe T, Li Z, Ford BD. Neuroprotection by neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Res. 2007. 1184:277–283.
49. Croslan DR, Schoell MC, Ford GD, Pulliam JV, Gates A, Clement CM, Harris AE, Ford BD. Neuroprotective effects of neuregulin-1 on B35 neuronal cells following ischemia. Brain Res. 2008. 1210:39–47.
50. Ni CY, Murphy MP, Golde TE, Carpenter G. gamma-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001. 294:2179–2181.
51. Lee HJ, Jung KM, Huang YZ, Bennett LB, Lee JS, Mei L, Kim TW. Presenilin-dependent gamma-secretase-like intramembrane cleavage of ErbB4. J Biol Chem. 2002. 277:6318–6323.
52. Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006. 9:1520–1525.
53. Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006. 314:664–666.
54. Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A. 2008. 105:5585–5590.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download