Abstract
Unipolar brush cells (UBCs) are excitatory interneurons with their somata located in the granular layer. Recently, T-brain factor 2 (Tbr2) was shown to be expressed in a subset of UBCs in mouse cerebellum. Scrambler mice exhibit severe cerebellum abnormalities, including the failure of embryonic Purkinje cell dispersal and a complete absence of foliation due to a mutation in the disabled-1 adaptor protein. Since most UBC markers are expressed postnatally, it has proven difficult to identify the relationship between developing Purkinje cell clusters and migrating UBCs. Because scrambler mice closely mimic normal embryonic day 18 cerebellum, we examined whether Tbr2-positive UBCs are associated with Purkinje cell cluster markers such as zebrin II, which is the most studied compartmentation marker in the cerebellum. We investigated the distribution of Tbr2-positive UBCs in this mutant by using anti-Tbr2 immunocytochemistry. The data revealed that Tbr2 immunoreactivity was exclusively present in the nucleus of UBCs in scrambler cerebellum. Based on expression data, a Tbr2-positive UBC map was constructed. In addition, Tbr2-positive UBCs are found associated with ectopic zebrin II-immunoreactive Purkinje cell clusters in scrambler cerebellum. These data suggest that UBCs use Purkinje cell compartmentation to migrate into their final position through interactions with the embryonic array of specific Purkinje cell subtypes.
Unipolar brush cells (UBCs) are excitatory interneurons with their somata located in the cerebellum of granular layer (Altman & Bayer, 1977; Floris et al., 1994; Mugnaini & Floris, 1994; Nunzi et al., 2002). They are characterized by a small (9~12 µm) soma with a single short dendritic shaft and brush-like dendritic processes (Altman & Bayer, 1977; Mugnaini & Floris, 1994). In mice, UBCs are mainly present in the posterior lobules (especially abundant in lobules IX and X, Nunzi et al., 2002). They receive main excitatory inputs from the primary and secondary vestibulocerebellar mossy fibers (extrinsic mossy fibers) and project axons (intrinsic mossy fibers) that innervate both granule cells and other UBCs (Nunzi & Mugnaini, 2000; Nunzi et al., 2001).
The mammalian cerebellum cortex is subdivided rostrocaudally and mediolaterally into a reproducible array of zones and stripes (for reviews see Hawkes & Gravel, 1991; Hawkes, 1997). The expression patterns of various Purkinje and granule cell antigens are restricted across zonal boundaries and have unique striped expression patterns within different zones (Hawkes & Eisenman, 1997; Ozol & Hawkes, 1997; Ozol et al., 1999). In addition, the abnormal cerebellum phenotypes of certain mutant mice are regionally restricted to particular zones (for reviews, see Armstrong and Hawkes, 2000; Eisenman, 2000). Among the compartmentation antigens in cerebellum, zebrin II is the most studied molecular marker. Zebrin II was first identified as the antigen that is recognized by a mouse monoclonal antibody produced by immunization with a crude cerebellum homogenate from the weakly electric fish Apteronotus (Brochu et al., 1990). Zebrin II was later shown to be an epitope on the respiratory isoenzyme aldolase c (Aldoc: Ahn et al., 1994). The zebrin II expression pattern consists of an array of parasagittal stripes displayed symmetrically on either side of the midline, each comprising a few hundred to a few thousand Purkinje cells.
T-brain factor 2 (Tbr2)-immunoreactive UBCs are expressed in a subset of UBCs (Englund et al., 2006). It has proven difficult to identify the relationship between developing Purkinje cell clusters and migrating UBCs, since most UBC markers are expressed postnatally. During mouse cerebellar development, early Purkinje cell clusters disperse along the rostrocaudal axis around birth. The reelin-disabled-1 signaling pathway is known to mediate this process. Scrambler mice lack the disabled-1 gene, resulting in the failure of embryonic Purkinje cell clusters to disperse (Sweet et al., 1996; Goldowitz et al., 1997; Sheldon et al., 1997: see also Howell et al., 1997). Because scrambler cerebellar cortex mimics the topography of embryonic day 18 cerebellum, we examined whether Tbr2-positive UBCs are associated with Purkinje cell cluster markers. In this study, we show that Tbr2-immunoreactive UBCs are associated with specific zebrin II-positive Purkinje cell clusters in the mutant. These data suggest that UBC somata become restricted to stripes through interactions with the embryonic specific Purkinje cell clusters.
Postnatal day 60 male scrambler mice (N=4) and normal littermates (N=3) were obtained from the Jackson Laboratory, Bar Harbor, Maine, USA. The littermate controls gave identical results to those from the two outbred strains. The experimental procedures used in this study were followed and approved by the Animal Care and Use Committee of Konyang University, and these are consistent with NIH (Bethesda, MD, USA) guidelines.
Anti-zebrin II is a mouse monoclonal antibody produced by immunization with a crude cerebellum homogenate from the weakly electric fish Apteronotus (Brochu et al., 1990): it was used directly from spent hybridoma culture medium at a concentration of 1 : 1,000. In the cerebellum, zebrin II immunoreactivity is restricted to a Purkinje cell subset (Brochu et al., 1990) together with weak uniform expression in some glial cells. Affinity purified rabbit anti-Tbr2 was raised against mouse Tbr2 synthetic peptide (Englund et al., 2006; Chemicon, Temecula, CA, USA, 1 : 1,000).
Mice were deeply anaesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.9% NaCl in 0.1M phosphate buffer (PBS: pH 7.4), followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). The brains were removed from the skull and post-fixed in 4% paraformaldehyde at 4℃ for 48 hours. The cerebellum was cryoprotected through a series of buffered sucrose solutions: 10% (2 hrs), 20% (2 hrs) and 30% (overnight). A series of 40 µm thick transverse sections were cut through the extent of the cerebellum on a cryostat and collected for free-floating immunohistochemistry. Briefly, tissue sections were washed thoroughly, blocked with 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and then incubated in 0.1M phosphate buffered saline (PBS) containing 0.1% Triton-X and the primary antibody for 16~18 hours at 4℃. Secondary incubation in horseradish peroxidase (HRP)-conjugated goat anti-rabbit or HRP-conjugated goat anti-mouse antibodies (all diluted 1 : 200 in PBS; Jackson ImmunoResearch Laboratories) was for 2 hours at room temperature. Diaminobenzidine (DAB, 0.5 mg/mL) was used to visualize the reaction product. Finally, sections were dehydrated through an alcohol series, cleared in xylene and cover-slipped with Entellan mounting medium (BDH Chemicals, Toronto, ON, Canada).
Cerebellum sections for fluorescent immunohistochemistry were processed. Tissue sections were washed, blocked in PBS containing 10% normal goat serum (Jackson ImmunoResearch Laboratories), incubated in both primary antibodies overnight at room temperature, rinsed, and then incubated for 2 hours at room temperature in a mixture of Alexa 546-conjugated goat anti-rabbit Ig and Alexa 488-conjugated goat anti-mouse Ig (Molecular Probes Inc., Eugene, OR, USA), all diluted 1 : 2,000. After several rinses in 0.1M PBS, sections were coverslipped in non-fluorescing mounting medium (Fluorsave Reagent, Calbiochem, La Jolla, CA, USA).
Photomicrographs were captured with a SPOT Cooled Color digital camera (Diagnostic Instruments Inc, Sterling Height, MI, USA) and assembled in Adobe Photoshop. The images were cropped and corrected for brightness and contrast, but not otherwise manipulated.
Recently, we reported that UBCs are normally associated with Purkinje cell clusters, both in the perinatal and postnatal cerebellum, and Purkinje cell topography governs the UBC cluster distribution (Chung et al., 2009). In this study, we applied a novel UBC marker and investigated its distribution in scrambler mutant cerebellum, in which Purkinje cells are located in ectopic clusters. In scrambler cerebellum, most anti-Tbr2 immunoreactivity is localized to a single interneuron type, the UBC (Fig. 1: Englund et al., 2006). Tbr2 immunoreactivity is exclusively restricted to the nucleus - the somata, dendrites and axons are not labeled (Fig. 1A inlet). No other cell type expresses Tbr2 in the cerebellum. Serial construction of the distribution of Tbr2-immunoreactive UBCs in scrambler cerebellum is demonstrated in Fig. 2. Interestingly in scrambler, UBCs are located ectopically. In posterior cerebellum, Tbr2-positive UBCs are mainly present in the lower part of the vermis (Fig. 2A). Also, note that a few UBCs are observed in the hemisphere (Fig. 2A). In the anterior part of the scrambler cerebellum, the distribution of UBCs is concentrated in the midline portion and dorsal hemisphere region (Fig. 2B, C).
To identify the relationship between Purkinje cell subsets in scrambler mice, transverse sections were double stained for anti-zebrin II and anti-Tbr2 (Fig. 3). The ectopic clusters showed both zebrin II-positive and Tbr2-positive phenotypes, and Tbr2 expression reveals subdivisions within the population of zebrin II-immunoreactive Purkinje cells (Fig. 3). Supporting this observation, anti-Tbr2 and anti-zebrin II double stained sections, from the adult scrambler cerebellum, clearly show that Tbr2-immunoreactive UBC clusters are associated with ectopic Purkinje cell clusters in the posterior cerebellum (Fig. 3C, F). These data suggest that ectopic Purkinje cells might directly influence the positioning of Tbr2-immunoreactive UBCs by forcing them into abnormal locations.
Though lineage relationships are largely unknown (Abbott & Jacobowitz, 1995), UBCs are likely to behave similar to other cerebellum interneurons, which are born in the subventricular zone of the fourth ventricle and migrate out into the white matter tracts. Originally, UBCs are thought to arise from the fourth ventricle; however, more recent data suggest that their origin might be the rhombic lip (Englund et al., 2006). The authors showed that rhombic lip ablation in slice cultures significantly reduced the number of Tbr2-positive UBCs. In addition, in Math1 (molecular marker for rhombic lip and its derivatives) null cerebellum, the production of Tbr2-positive UBCs is decreased as well (Englund et al., 2006). These data suggest that at least some populations of UBCs originate from the rhombic lip. UBCs are born earlier than cerebellum inhibitory interneurons and migrate into the developing cerebellum anlage shortly after deep cerebellum neurons and Purkinje cells (immature UBCs have been described in the mouse cerebellum as early as embryo age (E) 14: Abbott and Jacobowitz, 1995). By late embryogenesis, they encounter Purkinje cells that are already organized as discrete clusters. During postnatal development, UBCs and Purkinje cell clusters remain associated until approximately P4. The data presented here for scrambler mice reveal an interaction between UBCs and Purkinje cell perinatal clusters that is preserved into adulthood due to the failure of reelin-disabled1 signaling.
Stripes of Purkinje cells form during cerebellum development through the dispersal of embryonic clusters, a process that requires the reelin-disabled-1 signaling pathway (Sweet et al., 1996; Goldowitz et al., 1997; Howell et al., 1997; Sheldon et al., 1997). In the mouse mutant scrambler, a lack of disabled-1 protein results in the failure of embryonic Purkinje cell cluster dispersal. Nevertheless, Chung et al., (2009) showed that in the scrambler cerebellum, careltinin-immunoreactive UBCs are found to be associated with ectopic zebrin II-immunopositive Purkinje cell clusters. In this study, we further demonstrate that a novel UBC marker, Tbr2-immunoreactive UBCs, are also found to be associated with zebrin II compartments. The anatomical significance is that the presence of Tbr2-positive UBCs is not just restricted to posterior lobules, but is also in anterior lobules. In scrambler, Tbr2-positive UBCs are seen in the hemisphere region suggesting that this novel set of UBCs uses a different migratory pathway during embryonic development since scrambler cerebellum represents E18 cerebellum. Taken together, the data show that the topography of UBCs is dramatically changed when Purkinje cells are located ectopically (scrambler), and this suggests that UBC somata become regionally restricted through interactions with the embryonic array of specific Purkinje cell subtypes. The mechanism that organizes the topography of the UBCs may mimic that for the mossy fibers. It has been suggested that mossy fiber topography is established through the interaction of their growth cones with the embryonic Purkinje cell topographical maps (e.g., reviewed in Sotelo & Chédotal, 2005). Mossy fibers are known to make direct contact with Purkinje cells as soon as they enter the cerebellum. Subsequently, mossy fiber growth cones detach from the Purkinje cells and make contacts in the granular layer - just beneath the Purkinje cells. This allows for the topographical relationship with the Purkinje cell compartments (Sotelo & Chédotal, 2005). The same may be the case for UBC topography, with UBCs behaving like mossy fiber growth cones: the embryonic Purkinje cell clusters would disperse into the adult array of stripes while taking the UBCs with them. This view is supported by the data on the distribution of UBCs in the scrambler. Since the scrambler cerebellum cortex represents the topography of the normal embryonic (E18) cerebellum, it is reasonable to speculate that the same relationship is true in the normal embryonic environment and that UBCs organize around embryonic Purkinje cell clusters. Thus, the simplest explanation is that the development of UBC topography is secondary to Purkinje cell compartmentation.
Figures and Tables
 | Fig. 1Unipolar brush cells (UBCs) express Tbr2 in the cerebellum of scrambler mice. Transverse scrambler cerebellum section through the posterior lobe vermis immunoperoxidase stained with anti-Tbr2. Very strong immunoperoxidase products were deposited in individual cells. Tbr2 immunoreactivity is exclusively detected in the nucleus of UBCs. No other structures are stained. The rectangular layer is demonstrated with high-power image in inlet. Midline is indicated by a dotted line. Scale bar = 125 µm. |
 | Fig. 2Serial reconstruction of the distribution of Tbr2-immunoreactive UBCs in scrambler mutant mice. (A) In posterior cerebellum, Tbr2-immunoreactive UBCs are mainly located in the ventral part of the vermis. (B~C) Middle and anterior part of the scrambler cerebellum. Tbr2-positive UBCs are present in the vermis and hemisphere region. Scale bar = 500 µm. |
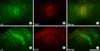 | Fig. 3A subset of Tbr2-immunoreactive UBCs is aligned with the zebrin II subset of Purkinje cells in the scrambler cerebellum. (A~C) Double immunofluorescence staining with anti-zebrin II and Tbr2 shows that ectopic Purkinje cells within the cerebellum express both zebrin II (green) and Tbr2 in scrambler vermis. (D~F) In Scrambler hemisphere, clusters of Tbr2-immunoreactive UBCs (red) are topographically aligned with a subset of zebrin II-immunoreactive Purkinje cells (green). Scale bar = 100 µm. |
References
1. Abbott LC, Jacobowitz DM. Development of calretinin-immunoreactive unipolar brush-like cells and an afferent pathway to the embryonic and early postnatal mouse cerebellum. Anat Embryol Berl. 1995. 191:541–559.
2. Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development. 1994. 120:2081–2090.
3. Altman J, Bayer SA. Time of origin and distribution of a new cell type in the rat cerebellar cortex. Exp Brain Res. 1977. 29:265–274.
4. Armstrong CL, Hawkes R. Pattern formation in the cerebellar cortex. Biochem Cell Biol. 2000. 78:551–562.
5. Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990. 291:538–552.
6. Chung SH, Sillitoe RV, Croci L, Badaloni A, Consalez G, Hawkes R. Purkinje cell phenotype restricts the distribution of unipolar brush cells. Neuroscience. 2009. 164:1496–1508.
7. Eisenman LM. Antero-posterior boundaries and compartments in the cerebellum: evidence from selected neurological mutants. Prog Brain Res. 2000. 124:23–30.
8. Englund C, Kowalczyk T, Daza RA, et al. Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. J Neurosci. 2006. 26:9184–9195.
9. Floris A, Dino M, Jacobowitz DM, et al. The unipolar brush cells of the rat cerebellar cortex and cochlear nucleus are calretinin-positive: a study by light and electron microscopic immunocytochemistry. Anat Embryol. 1994. 189:495–520.
10. Goldowitz D, Cushing RC, Laywell E, et al. Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin. J Neurosci. 1997. 17:8767–8777.
11. Hawkes R. An anatomical model of cerebellar modules. Prog Brain Res. 1997. 114:39–52.
12. Hawkes R, Eisenman LM. Stripes and zones: the origins of regionalization of the adult cerebellum. Perspect Dev Neurobiol. 1997. 5:95–105.
13. Hawkes R, Gravel C. The modular cerebellum. Prog Neurobiol. 1991. 36:309–327.
14. Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997. 389:733–737.
15. Mugnaini E, Floris A. The unipolar brush cell: a neglected neuron of the mammalian cerebellar cortex. J Comp Neurol. 1994. 339:174–180.
16. Nunzi MG, Birnstiel S, Bhattacharyya BJ, Slater NT, Mugnaini E. Unipolar brush cells form a glutamatergic projection system within the mouse cerebellar cortex. J Comp Neurol. 2001. 434:329–341.
17. Nunzi MG, Mugnaini E. Unipolar brush cell axons form a large system of intrinsic mossy fibers in the postnatal vestibulocerebellum. J Comp Neurol. 2000. 422:55–65.
18. Nunzi MG, Shigemoto R, Mugnaini E. Differential expression of calretinin and metabotropic glutamate receptor mGluR1 alpha defines subsets of unipolar brush cells in mouse cerebellum. J Comp Neurol. 2002. 451:189–199.
19. Ozol KO, Hawkes R. compartmentation of the granular layer of the cerebellum. Histol Histopathol. 1997. 12:171–184.
20. Ozol K, Hayden JM, Oberdick J, Hawkes R. Transverse zones in the vermis of the mouse cerebellum. J Comp Neurol. 1999. 412:95–111.
21. Sheldon M, Rice DS, D'Arcangelo G, et al. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997. 389:730–733.
22. Sotelo C, Chédotal A. Development of the olivocerebellar system: migration and formation of cerebellar maps. Prog Brain Res. 2005. 148:1–20.
23. Sweet HO, Bronson RT, Johnson KR, Cook SA, Davisson MT. Scrambler, a new neurological mutation of the mouse with abnormalities of neuronal migration. Mamm Genome. 1996. 7:798–802.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download