Abstract
Nitric Oxide (NO) actively participates in the regulation of neuronal intracellular Ca2+ levels by modulating the activity of various channels and receptors. To test the possibility that modulation of Ca2+ buffer protein expression level by NO participates in this regulatory effect, we examined expression of calbindin-D28k, calretinin, and parvalbumin in the cerebellum of neuronal NO synthase knock-out (nNOS(-/-)) mice using immunohistochemistry. We observed that in the cerebellar cortex of the nNOS(-/-) mice, expression of calbindin-D28k and parvalbumin were significantly increased while expression of calretinin was significantly decreased. These results suggest another mechanism by which NO can participate in the regulation of Ca2+ homeostasis.
Nitric Oxide (NO) is not only a neurotransmitter but also a neuromodulator which exerts many functions in the nervous system (Esplugues, 2002; Rhyu et al., 2003; Abbott & Nahm, 2004). Since NO can cross the lipid bilayer freely and has a very short lifespan, neurons cannot sequester NO nor regulate its local concentration (Dawson & Dawson, 1996). Therefore, the key to regulating NO in the brain is to control NO synthesis by regulating the activity of neuronal NO synthase (nNOS). This regulation of NO synthesis is mainly mediated by cytosolic Ca2+ levels. The Ca2+ influx from extracellular fluid and the release of Ca2+ from intracellular stores increase Ca2+ concentrations in the neuronal cytoplasm. Increased Ca2+ binds calmodulin (CaM) and then the Ca2+CaM complex activates nNOS by direct binding. If the Ca2+ concentration falls, it dissociates from CaM, which in turn dissociates from nNOS resulting in nNOS deactivation (Knowles et al., 1989; Sheng et al., 1992).
While the synthesis of NO is regulated by Ca2+, NO can also influence Ca2+ levels in neuronal cytoplasm. NO diminishes activity of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) (Lei et al., 2000) and N-methyl-D-aspartate (NMDA)-type glutamate receptor (Lei et al., 1992; Manzoni et al., 1992). NO inhibits voltage-gated Ca22+channels such as L-type (Doerner & Alger, 1988) and N-type Ca2+ channels (Yoshimura et al., 2001). The increase of Ca2+ concentration through these receptors and channels can be reduced by these means. Not only Ca2+ influx from extracellular fluid but also Ca2+ release from intracellular Ca2+ stores are modulated by NO. NO induces ryanodine receptor phosphorylation through protein kinase G, which results in increased Ca2+ release from the endoplasmic reticulum into the cytoplasm (Clementi et al., 1996). Therefore it can be said that NO actively participates in the regulation of Ca2+ homeostasis of neurons.
The entire neuronal Ca2+ homeostasis regulation system consists of a Ca2+ entry system, intracellular Ca2+ store, Ca2+ extrusion system, and Ca2+ buffer. It can be hypothesized that NO participates in the regulation of Ca2+ homeostasis through mechanisms other than modulating the Ca2+ entry system and intracellular Ca2+ store. Previously it was shown that Ca2+ binding proteins (CaBPs) such as calbindin-D28k (CB) (Geula et al., 1993; Bertini et al., 1996) and calretinin (CR) (Arévalo et al., 1993) colocalize with nNOS in some populations of neurons. Similar cerebellar function defects are detected in both nNOS (Nelson et al., 1995) and CaBP knock-out mice (Airaksinen et al., 1997; Cheron et al., 2000). Based upon these findings, Ca2+ buffer may be a candidate for Ca2+ homeostasis regulation by NO. It is well known that CaBPs such as CB, CR, and parvalbumin (PV) act as Ca2+ buffers in neurons (Schwaller et al., 2002) and that nNOS and these proteins are abundantly expressed and exert several functions in the cerebellum (Nelson et al., 1995; Schwaller et al., 2002). Therefore, to test NO's influences on these Ca2+ buffer proteins, we examined changes in their expression in the cerebellum of nNOS knock-out mice (nNOS(-/-) mice) (Huang et al., 1993) using immunohistochemistry. We were able to demonstrate specific changes in expression of each Ca2+ buffer protein in the cerebellum of the nNOS(-/-) mice.
Male mice 3~4 months old were utilized for this study. There were 12 C57BL/6 controls and 10 nNOS(-/-) B6, 129S-Nos1tm1Pih obtained from Dr. Oh (Induced Mutant Resources Program, Genetic Resources Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea). All animals were treated in accordance with the 'Principles of Laboratory Animal Care' (NIH publication No. 86~23, revised in 1985). The mice were perfused transcardially with cold phosphate buffered saline (PBS, 0.05M, pH 7.4), followed by ice-cold 4% paraformaldehyde. The brains were cryoprotected in a series of cold sucrose solutions, and cut at 40 µm in the coronal plane. Immunohistochemistry was performed in accordance with the free-floating method described earlier (Chung et al., 2000). Rabbit anti-CB polyclonal antibody, rabbit anti-CR polyclonal antibody, and anti-PV monoclonal antibody (AB1778 and AB5054; Chemicon International, Temecula, CA, USA for CB and CR respectively and P3088; Sigma, Saint Louis, MI, USA for PV) were used as primary antibodies.
A sample of sections was reacted without primary antiserum, and different samples were exposed to primary antiserum that had been preabsorbed for 24 hours with control antigen peptides. Sections from these samples did not exhibit any immunoreactivity as described in this report (Fig. 1). We randomly selected 5 unit areas at each region in the cerebellum from control (n=12) and nNOS(-/-) mice (n=10) and calculated the numbers of CB, CR, and PV-ir per unit area. Sections from each control and nNOS(-/-) group were stained together eliminating the variable of different experimental conditions. Visual assessment and densitometric measurements using an NIH image program (Scion Image) were evaluated to determine staining density. The t-test was used to investigate whether changes in CaBPs expression were statistically significant (*P<0.01).
Immunohistochemistry images were taken using a ProgRes C14 digital camera with ProgRes C14 software (JENOPTIC Laser, Optic, System, Munich, Germany). Image-editing software (Adobe Photoshop) was used to adjust size and contrast and combine the images obtained.
In the cerebellar cortex, each CaBP showed a characteristic expression pattern. CB-immunoreactivity was observed in the neuropil of the molecular layer and cell bodies of Purkinje cells (Fig. 2A) whereas CR-immunoreactivity was confined to neuropil of the molecular layer and granular cells of the granular layer (Fig. 3A). PV was expressed by the neuropil, cells of the molecular layer, and cell bodies of Purkinje cells (Fig. 4A). These expression patterns of CB, CR, and PV were well preserved in the nNOS(-/-) mice (Fig. 2A, B for CB; Fig. 3A, B for CR; Fig. 4A, B for PV). However, the immunoreactivity of each CaBP was significantly changed. In all layers of the cerebellar cortex, CB-immunoreactivity was significantly increased (Fig. 2A, B, E) and in contrast to CB, CR-immunoreactivity was significantly decreased (Fig. 3A, B, E). Regarding PV, PV-immunoreactivity of the molecular layer and the Purkinje cell layer were significantly increased whereas that of the granular layer was significantly decreased (Fig. 4A, B, E).
Immunoreactivity of CB and CR in the deep cerebellar nuclei was much lower than that in the cerebellar cortex and most were confined in neuropil (Fig. 2C, 3C). Similar to changes in the cerebellar cortex, CB-immunoreactivity of the nNOS(-/-) mice was significantly increased (Fig. 2D, E) and CR-immunoreactivity of the nNOS(-/-) mice was decreased, although this change was not statistically significant (Fig. 3D, E). In contrast to CB and CR, relatively high levels of PV were observed in the deep cerebellar nuclei and expressed not only in neuropil but also in cell bodies (Fig. 4C). Immunoreactivity was similar between control and nNOS(-/-) groups (Fig. 4C, D, E) but cell bodies of nNOS(-/-) mice appeared to express higher PV. (Fig. 4C, D).
Previously the distribution of CB, CR, and PV in the cerebellum was carefully examined and their characteristic localizations reported (Celio, 1990; Résibois & Rogers, 1992). In the molecular layer, stellate cells and basket cells expressed PV, parallel fibers of granule cells expressed CR, and dendrites of Purkinje cells expressed CB and PV. In the Purkinje cell layer the cell bodies of Purkinje cells showed CB and PV-immunoreactivity. In the granular layer only CR was expressed by granular cells. These characteristic expressions of CB, CR, and PV match well with our results (Fig. 2~4). Therefore CB and CR-immunoreactivity in neuropil of the molecular layer are thought to be due to dendrites of Purkinje cells and parallel fibers of granule cells respectively. PV-immunoreactivity of cells and neuropil of the molecular layer may be due to stellate cells, basket cells, and dendrites of Purkinje cells.
The CB, CR, and PV actively participate in the regulation of neuronal intracellular Ca2+ levels by binding Ca2+ and lowering its concentration. The intracellular steady state Ca2+ level of neurons is not affected by these CaBPs (Chard et al., 1993; Schwaller et al., 2002), because it is determined by Ca2+ uptake and extrusion system which remains functional until Ca2+ has attained its steady state level. Instead, these calcium buffer proteins modulate the temporal and spatial properties of intracellular Ca2+ distribution. For example, CB significantly reduces the amplitude of the Ca2+ transient and slows down the decay of Ca2+ levels in cerebellar Purkinje cells (Airaksinen et al., 1997). PV, which has slower Ca2+ binding properties than CB, increases the initial rate of decay of Ca2+ and subsequently prolongs its late phase in bovine chromaffin cells (Lee et al., 2000). Therefore, it is possible that NO influences intracellular Ca2+ levels of the cerebellar neurons by modulating CaBPs expression levels.
The nNOS(-/-) mice have no evident defects in usual locomotor activity (Huang et al., 1993) but show discrete abnormalities in balance and motor coordination selectively at night (Kriegsfeld et al., 1999). Similarly, CB knock-out mice (Airaksinen et al., 1997) and Purkinje cell-specific conditional CB knock-out mice (Barski et al., 2000) show normal motor functions in usual environments but slip when transversing narrow runways, which force them to change and adapt their stride. Also in CR knock-out mice, abnormal Purkinje cell activity in the cerebellum and abnormal wheel running test results are observed whereas other motor functions remain normal (Schanne et al., 1979; Cheron et al., 2000). Regarding these findings, the nNOS(-/-) mice, the CB knock-out mice, and the CR knock-out mice seem to have very similar cerebellar functional defects, although motor function tests used are not exactly the same. This suggests that there may be a common underlying mechanism. Since altered CB and CR expression in the nNOS(-/-) mice was observed in this study, altered expression of these proteins and resulting temporal and spatial intracellular Ca2+ distribution property changes may be good candidates for that common mechanism.
It is well known that intracellular concentration of Ca2+ and the Ca2+-dependent signaling system including NOS are closely related to neuronal degenerations (Schanne et al., 1979; Lipton et al., 1993). The Ca2+ buffering property of CB, CR, and PV allows for the hypothesis that these buffers may have neuroprotective effects. There is some in vitro data supporting this hypothesis (D'Orlando et al., 2001; D'Orlando et al., 2002). In spinocerebellar ataxia type I, it is suggested that down-regulation of CB and PV leads to cerebellar Purkinje cell death (Vig et al., 2000). Many studies have also shown results against this hypothesis (Kuźnicki et al., 1996; Airaksinen et al., 1997; Bouilleret et al., 2000). In CB, CR, and PV knock-out mice there is no evidence of abnormal neuronal loss (Schwaller et al., 2002), resulting in doubts as to a generalized neuroprotective role for these proteins. Like the Ca2+ buffer proteins, no unusual neuronal death is observed in the nNOS(-/-) mice (Huang et al., 1993; Nelson et al., 1995); therefore, it is not obvious that Ca2+ buffer proteins such as CB, CR, and PV have generalized neuroprotective effects. Their modulated expression by NO seems to have minor effects on neuronal survival.
In the present study, we demonstrated that expression levels of CB, CR, and PV are characteristically and significantly altered in the cerebellum of nNOS(-/-) mice using immunohistochemistry techniques (Figs. 2~4). These changing patterns were preserved in all animals studied. Regarding these findings, it can be concluded that NO modulates CaBP expression in neurons of the cerebellum, and by this means, NO participates in Ca2+ homeostasis and regulation of neurons. As expression patterns of these proteins are not changed, it appears that NO influences only the expression level, not the expression pattern in the cerebellum.
For the first time, we demonstrated that NO specifically modulates the expression of Ca2+ buffer proteins such as CB, CR, and PV in the cerebellum. This result suggests another mechanism by which NO participates in the regulation of Ca2+ homeostasis. Since modulation of expression levels of Ca2+ buffer proteins can influences temporal and spatial properties of intracellular Ca2+ distribution, it appears that NO can exerts its various functions not only in the cerebellum but also in the other parts of the brain. The exact mechanism of this regulation and its functional significance requires further elucidation.
Figures and Tables
 | Fig. 1Tests for specificity of primary antibodies (A, B). A sample of sections reacted without primary antiserum (A), and a different sample exposed to primary antiserum preabsorbed for 24 hours with CB (B) do not exhibit any immunoreactivity. No immunoreactivity is observed in both samples exposed to primary antiserums preabsorbed for 24 hours with CR and PV (data not shown). Scale bar = 150 µm. |
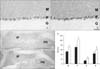 | Fig. 2Changes of CB-immunoreactivity in the cerebellum of nNOS(-/-) mice (B, D), compared with that of the control mice (A, C). In control mice, CB-immunoreactivity is observed in Purkinje cells, neuropil of the molecular layer of the cerebellar cortex (A) and neuropil of the deep cerebellar nuclei (C). This expression pattern is preserved in the nNOS(-/-) mice (B, D) but in all cerebellar cortical layers (B) and deep cerebellar nuclei (D), CB-immunoreactivity is increased. In image analysis (E), these differences are statistically significant. Mean density is the sum of the white values of all the pixels in the selection divided by the number of pixels within the selection. Values are the mean±standard deviations. A t-test was performed (*P<0.01). D, deep cerebellar nuclei; FN, fastigial nucleus; G, granular layer; IP, interposed nucleus; M, molecular layer; P, Purkinje cell layer. Scale bar (A, B) = 100 µm; (C, D) = 400 µm. |
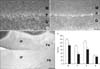 | Fig. 3Decreased CR expression in the cerebellum of nNOS(-/-) mice (A, C for the control mice and B, D for the nNOS(-/-) mice). In the cerebellar cortex of both group, granule cells and neuropil of the molecular layer express CR but Purkinje cells express little CR (A, B). Compared with control mice (A), CR-immunoreactivity is significantly decreased in all cerebellar cortical layers of nNOS(-/-) mice (B, E). In contrast to the cerebellar cortex, CR-immunoreactivity of the deep cerebellar nuclei is very low (C, D). In many animals CR expression seems to be decreased in these regions in the nNOS(-/-) mice although their differences are not statistically significant (E). Mean density is the sum of the white values of all the pixels in the selection divided by the number of pixels within the selection. Values are the mean±standard deviations. A t-test was performed (*P<0.01). The graph in E shows results from image analysis. D, deep cerebellar nuclei; FN, fastigial nucleus; G, granular layer; IP, interposed nucleus; M, molecular layer; P, Purkinje cell layer. Scale bar (A, B) = 100 µm; (C, D) = 400 µm. |
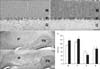 | Fig. 4Changes of PV-immunoreactivity in the cerebellum of the nNOS(-/-) mice (B, D) compared with that of control mice (A, C). In the cerebellar cortex of the control mice, the Purkinje cells, neuropil, and cells of the molecular layer express PV (A). Although PV expression patterns of nNOS(-/-) mice are similar to that of control mice, PV-immunoreactivity of Purkinje cells and the molecular layer is significantly increased (A, B, E) and that of the granular layer is significantly decreased (A, B, E) in nNOS(-/-) mice. In the deep cerebellar nuclei of the control and the nNOS(-/-) mice, cell bodies as well as neuropil express PV (C, E). The change of immunoreactivity was minor but cell bodies of the nNOS(-/-) mice seemed to express higher PV-immunoreactivity (C~E). The graph in E shows results from image analysis. Mean density is the sum of the white values of all pixels in the selection divided by the number of pixels within the selection. Values are the mean±standard deviations. A t-test was performed (*P<0.01). D, deep cerebellar nuclei; FN, fastigial nucleus; G, granular layer; IP, interposed nucleus; M, molecular layer; P, Purkinje cell layer. Scale bar (A, B) = 100 µm; (C, D) = 400 µm. |
References
1. Abbott LC, Nahm SS. Neuronal nitric oxide synthase expression in cerebellar mutant mice. Cerebellum. 2004. 3:141–151.
2. Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA. 1997. 94:1488–1493.
3. Airaksinen MS, Thoenen H, Meyer M. Vulnerability of midbrain dopaminergic neurons in calbindin-D28k-deficient mice: lack of evidence for a neuroprotective role of endogenous calbindin in MPTP-treated and weaver mice. Eur J Neurosci. 1997. 9:120–127.
4. Arévalo R, Sánchez F, Alonso JR, Rubio M, Aijón J, Vázquez R. Infrequent cellular coexistence of NADPH-diaphorase and calretinin in the neurosecretory nuclei and adjacent areas of the rat hypothalamus. J Chem Neuroanat. 1993. 6:335–341.
5. Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000. 28:93–98.
6. Bertini G, Peng ZC, Bentivoglio M. The chemical heterogeneity of cortical interneurons: nitric oxide synthase vs. calbindin and parvalbumin immunoreactivity in the rat. Brain Res Bull. 1996. 39:261–266.
7. Bouilleret V, Schwaller B, Schurmans S, Celio MR, Fritschy JM. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium-binding proteins parvalbumin, calbindin, or calretinin. Neuroscience. 2000. 97:47–58.
8. Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990. 35:375–475.
9. Chard PS, Bleakman D, Christakos S, Fullmer CS, Miller RJ. Calcium buffering properties of calbindin D28k and parvalbumin in rat sensory neurones. J Physiol. 1993. 472:341–357.
10. Cheron G, Schurmans S, Lohof A, et al. Electrophysiological behavior of Purkinje cells and motor coordination in calretinin knock-out mice. Prog Brain Res. 2000. 124:299–308.
11. Chung YH, Shin C, Park KH, Cha CI. Immunohistochemical study on the distribution of neuronal voltage-gated calcium channels in the rat cerebellum. Brain Res. 2000. 865:278–282.
12. Clementi E, Riccio M, Sciorati C, Nisticó G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. Role of the nitric oxidecGMP pathway. J Biol Chem. 1996. 271:17739–17745.
13. Dawson VL, Dawson TM. Nitric oxide actions in neurochemistry. Neurochem Int. 1996. 29:97–110.
14. Doerner D, Alger BE. Cyclic GMP depresses hippocampal Ca2+ current through a mechanism independent of cGMP-dependent protein kinase. Neuron. 1988. 1:693–699.
15. D'Orlando C, Celio MR, Schwaller B. Calretinin and calbindin D-28k, but not parvalbumin protect against glutamate-induced delayed excitotoxicity in transfected N18-RE 105 neuroblastoma-retina hybrid cells. Brain Res. 2002. 945:181–190.
16. D'Orlando C, Fellay B, Schwaller B, et al. Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 2001. 909:145–158.
17. Esplugues JV. NO as a signaling molecule in the nervous system. Br J Pharmacol. 2002. 135:1079–1095.
18. Geula C, Schatz CR, Mesulam MM. Differential localization of NADPH-diaphorase and calbindin-D28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience. 1993. 54:461–476.
19. Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993. 75:1273–1286.
20. Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA. 1989. 86:5159–5162.
21. Kriegsfeld LJ, Eliasson MJ, Demas GE, et al. Nocturnal motor coordination deficits in neuronal nitric oxide synthase knock-out mice. Neuroscience. 1999. 89:311–315.
22. Kuźnicki J, Isaacs KR, Jacobowitz DM. The expression of calretinin in transfected PC12 cells provides no protection against Ca(2+)-overload or trophic factor deprivation. Biochem Biophys Acta. 1996. 1313:194–200.
23. Lee SH, Schwaller B, Neher E. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: implications for [Ca2+] transients of neuronal dendrites. J Physiol. 2000. 525:419–432.
24. Lei S, Jackson MF, Jia Z, et al. Cyclic GMP-dependent feedback inhibition of AMPA receptors is independent of PKG. Nat Neurosci. 2000. 3:559–565.
25. Lei SZ, Pan ZH, Aggarwal SK, et al. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992. 8:1087–1099.
26. Lipton SA, Choi YB, Pan ZH, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993. 364:626–632.
27. Manzoni O, Prezeau L, Marin P, Deshager S, Bockaert J, Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992. 8:653–662.
28. Nelson RJ, Demas GE, Huang PL, et al. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995. 378:383–386.
29. Résibois A, Rogers JH. Calretinin in rat brain: an immunohistochemical study. Neuroscience. 1992. 46:101–134.
30. Rhyu IJ, Nahm SS, Hwang SJ, et al. Altered neuronal nitric oxide synthase expression in the cerebellum of calcium channel mutant mice. Brain Res. 2003. 977:129–140.
31. Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979. 206:700–702.
32. Schiffmann SN, Cheron G, Lohof A, et al. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. Proc Natl Acad Sci USA. 1999. 96:5257–5262.
33. Schwaller B, Meyer M, Schiffmann S. 'New' functions for 'old' proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002. 1:241–258.
34. Sheng H, Schmidt HH, Nakane M, et al. Characterization and localization of nitric oxide synthase in non-adrenergic non-cholinergic nerves from bovine retractor penis muscles. Br J Pharmacol. 1992. 106:768–773.
35. Vig PJ, Subramony SH, Qin Z, McDaniel DO, Fratkin JD. Relationship between ataxin-1 nuclear inclusions and Purkinje cell specific proteins in SCA-1 transgenic mice. J Neurol Sci. 2000. 174:100–110.
36. Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001. 86:304–331.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download