Introduction
Alzheimer's disease (AD) is most common cause of dementia in elderly individuals. Brain regions involved in learning, memory, and emotional behaviors (namely, the entorhinal cortex, hippocampus, basal forebrain, and amygdala) are reduced in size in AD patients as a result of the degeneration of synapses and ultimately, the death of neuron (Cutler et al., 2004). The molecular pathological hallmarks of AD are intracellular neurofibrillary tangles and extracellular amyloid (Aβ) plaques. Despite the influence of various genetic and environmental factors, as well as the aging process on the manifestation of AD, multiple lines of evidence from studies in experimental models and in AD brain tissue have demonstrated that the underlying neurodegeneration in AD is associated with morphological and biochemical features of apoptosis. Human studies have shown significant synaptic pathology in AD (Gonatas et al., 1967) and have identified synapse loss as a major correlate of cognitive impairment in the disease (Terry et al., 1991). Among the mechanisms of cell death, apoptosis has been proposed to explain the cell loss observed in many neurodegenerative disorders (Sathasivam et al., 2001). There are two major pathways of apoptosis, namely, the intrinsic pathway and the extrinsic pathway. In the intrinsic pathway, the interaction between the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax plays a key role in the activation of apoptotic signals involving mitochondria which secondary release cytochrome C. Subsequently, the initiator caspase-9 is activated and can initiate the activation of executioner caspase, mainly caspase-3. The activated caspase-3 then leads to cell destruction by proteolysis (Sathasivam et al., 2001).
Neuregulins (NRGs) are highly expressed in the nervous system, where ErbB4 (an NRG receptor) is expressed at high levels in neurons during brain development (Marchionni et al., 1993). Studies of mice with targeted mutations revealed an essential role of NRGs in cardiac and neural crest-derived cell population development (Meyer & Birchmeier, 1995). NRG1 and its receptor ErbB tyrosine kinase are expressed not only in the developing nervous system, but also in the adult brain. Further, NRG1 function is largely mediated by a class of receptor tyrosine kinases including ErbB2, ErbB3, and ErbB4. Among the ErbB receptors, ErbB4 has been suggested to be the primary mediator of NRG1 function in the CNS. Recent biochemical studies indicated that ErbB4 is highly enriched in the postsynaptic density (PSD) of excitatory synapses (Garcia et al., 2000; Huang et al., 2000; Li et al., 2007a) and GABAergic presynaptic terminals in the cerebral cortex (Woo et al., 2007). Additionally, in the hippocampus, NRG1 mRNA is highly expressed in CA3 area, a region presynaptic to CA1 which exhibits ErbB4 expression (Law et al., 2004; Woo et al., 2007).
Moreover, recent studies have indicated that NRG1 can be neuroprotective for cortical neurons (Li et al., 2003), motor neurons (Ricart et al., 2006), dopaminergic neurons (Zhang et al., 2004), cochlear sensory neurons (Stankovic et al., 2004) and PC12 cells (Goldshmit et al., 2001; Di Segni et al., 2005) it has also exhibited neuroprotection following ischemia (Shyu et al., 2004; Li et al., 2007b; Croslan et al., 2008). These findings suggest that NRG1/ErbB4 signaling might be important in cognition, learning and memory formation through the modulation of synaptic plasticity and neuronal survival and is, therefore, a critical molecule in neurodegenerative disease. In this study we investigate whether ErbB4 immunoreactivity correlate to the apoptotic signing in AD.
Materials and Methods
Reagents and antibodies
ErbB4 (sc-283, sc-8050) antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Bax (#2774) human specific antibody was purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). Fluorescein avidin D (A-2001), Rhodamine avidin D (A-2002), avidin-biotin blocking solution (SP-2001), Vectashield (H-1000), Vector NovaRED peroxidase substrate kit (SK-4800), Biotinylated anti-rabbit IgG (BA-1000) and Biotinylated anti-mouse IgG (BA-2001) were supplied by Vector Laboratories (Burlingame, CA, USA). Hoechst 33342 (bis-Benzimide H33342 trihydrochloride, 14533) and DAB (3,3'-Diaminobenzidine tetrahydrochloride, D-5905) were supplied by Sigma Aldrich (St. Louis, MO, USA). TUNEL kit (ApoTaq plus peroxidase in Situ apoptosis detection kit, S7101) was purchased from Millipore Corporation (Billerica, MA, USA).
Human tissues preparation
All human brains (6 age-matched control and 10 Alzheimer brains) used for this study were generous gifts from Dr. Roger A. Brumback (School of Medicine, Creighton University, Omaha, NV, USA). They were obtained from autopsy within 24 postmortem hours and were diagnosed at the Alzheimer Center of the OUHSC (Oklahoma City, OK, USA). All experimental procedures were performed in accordance with 'The Guidelines of the Ethics Committee at Eulji University'. Immediately after removal, all brains were fixed within 10% neutral buffered formaline, and then prepared according to routine histological procedures for paraffin sectioning. The sections, 10 µm in thickness, were deparaffinized and hydrated according to routine graded xylene-alcohol methods. Hematoxyline and eosin staining for general histopathology and modified silver staining by Campbell et al. (1987) for detecting senile plaques and NFTs were employed. The detailed data for each brain has been summarized in Table 1.
TUNEL staining
For the best quality of staining, the following TUNEL method is usually applied to formalin-fixed, paraffin embedded brain tissue. The TUNEL method was done as follows. After dewaxing and hydration, the sections were washed in phosphate buffered saline (PBS, pH 7.6) for 5 minutes (min), and were treated with Proteinase K (20 µg/ml) diluted in PBS at room temperature (RT) for 15 min. They were then washed in distilled water (DW) for 5 min. The TUNEL incubation solution was prepared in double distilled water (DDW) according to the manufacture's protocol (Millipore). This solution was comprised of TdT buffer, cobalt chloride, TdT and biotin-16dUTP. The sections were incubated in TdT buffer for 1 hour (hr) at 37℃. After the incubation, brain sections were applied with stop solution for 10 min and washed twice in the PBS (each for 5 min). The sections were then incubated with anti-digoxigenin antibody peroxidase conjugate at RT for 30 min. Finally, brain sections were incubated in the DAB solution for 1 min. The fragmented DNAs were visualized as a brownish color inside nuclei. The sections were counter-stained with methyl green before being dehydrated and cleared through graded alcohols and xylenes, and cover-slipped. Images were captured with a Axiocam digital camera (MRC; Carl Zeiss Inc., Göttingen, Germany) attached on the Olympus AX70 microscope.
Immunohistochemical staining
Immunohistochemical analysis of ErbB4 was performed using a biotin-avidin method. The antibody reaction was visualized using 3,3-diaminobenzidine tetrahydrochloride plus hydrogen peroxide. The double immunostaining for ErbB4 and Bax was carried out by repeating two cycles of the indirect immunostaining as reported previously (Ezaki 2000). Appropriate combinations for color reactions were Ni-DAB (gray) for ErbB4 versus NovaRed (Red) for second markers. Images were captured with an Axiocam (MRC; Carl Zeiss Inc., Göttingen, Germany). In order to retrieve antigenecity, dewaxed sections were boiled within 0.1 mol/L citrate-buffered saline (pH 6.0) for 10 min. After cooling down for 30 min, the sections were rinsed in PBS. The endogenous peroxidase was quenched by 1% hydrogen peroxide in 10% methanol for 30 min. After two changes of PBS-T (0.1% Triton X-100 in 0.1 mol/L PBS, pH 7.6) washing for 5 min respectively, the sections were blocked for 1 hr in blocking solution (5% host serum +1% BSA in PBS-T) and incubated in primary antibody (anti-ErbB4, 1 : 200 or anti-Bax, 1 : 200) at 4℃ overnight. After PBS-T rinses, the sections were incubated in a biotinylated secondary antibody for 1 hr at RT. After PBS rinsing and an avidin-biotin-peroxidase complex (Vectastain Elite ABC kit) treatment for 1 hr at RT, the sections were developed for 5 min in a 0.05% DAB solution. As a negative control for nonspecific staining, the sections were incubated with initial incubation media minus the primary antibody, and otherwise processed as described. Images were captured with a Axiocam digital camera attached on the Olympus AX70 microscope.
Confocal immunofluorescence
Immunofluorescence was performed as previously described (Murata & Dalakas, 1999). In brief, the prestaining process was the same as above. Sections were incubated overnight at 4℃ in blocking solution containing rabbit anti-Bax (1 : 50) and after washing, with the appropriate secondary biotinylated antibody. After rinsing, the sections were incubated with rhodamine avidin D. Normal horse serum was then applied for 1 hr to block nonspecific background staining. Then sections were incubated with avidin-biotin blocking solution (Vector Laboratories) and incubated overnight at 4℃ in blocking solution containing rabbit anti-ErbB4 (1 : 50). Following this, they were washed and incubated with the appropriate secondary biotinylated antibody and after rinsing, the sections were incubated with FITC avidin D. The sections were then incubated with Hoechst 33342 for 30 min and mounted. The images were visualized using Bax with rhodamine (red) fluorescence, the ErbB4 with FITC (green) fluorescence, and Hoechst 33342 (blue) with UV fluorescence on the same sections, using a LSM 510 meta system (Zeiss LSM 510 laser scanning microscope, Carl Zeiss, Germany).
Statistical analysis
Within each region, two images were captured at evenly spaced intervals to represent the entire anatomic area of interest. All histological preparations were analyzed by a blinded investigator with the aid of Image J software (NIH). For multiple group comparisons, statistical differences were calculated using one-way ANOVA followed by Bonferroni's test. For comparison of means from the same group of cells, a Student's paired t-test was used. Values of P<0.05 were considered significant.
Results
Immunohistochemical diagnosis in AD hippocampus
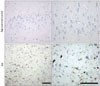
All brains (control and AD) were assigned Braak scores using neuropathological staging by Braak and Braak (Braak & Braak, 1991). The two braak groups are referred to as age-matched control (Braak stage 0, n=6) or AD (Braak stage I/V, n=10), referring to the extent of NFT and senile plaque in the hippocampus. Postmortem examination confirmed the diagnosis of AD given the because the presence of abundant modified Campbell silver stained amyloid deposits and NFT; H&E and LFB-CV staining also demonstrated marked cell loss (Fig. 1).
Apoptosis within the hippocampus of AD brains
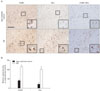
The TUNEL staining was employed to detect apoptotic cells within the hippocampus of AD brains. As shown in Fig. 2, with AD progression, the number of TUNEL positive hippocampal pyramidal neuron was significantly increased compared to the age-matched control. No TUNEL-positive pyramidal neuron is observed in hippocampal sectors of age-matched control.
The level of expression of ErbB4 in the apoptotic neurons of AD brains
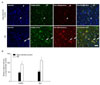
ErbB4 transcripts have been detected in the hippocampus of adult rat brain (Woo et al., 2007). To assess whether the level of expression of ErbB4 was altered in the hippocampus of AD human brain, we stained hippocampal sections of human brain. Neuronal staining was observed in hippocampal CA1 in 10 patients with a pathologically confirmed diagnosis of AD as well as in 6 cognitively normal age-matched controls. To determine whether expression of ErbB4 was altered in the hippocampal pyramidal neurons of human AD brains, we examined ErbB4 expression using both immunostaining and immunofluorescence methods. As indicated in Figs. 3A, B, 4A and B, ErbB4 immunoreactivity was significantly increased in the AD brain as compared to age-matched controls. At high magnification, the signal was revealed in pyramidal neurons. Programmed cell death or apoptosis, the critical process for neurodegeneration, involves multiple signaling pathways as well as the crucial modulators of cell survival, the Bcl-2 family of proteins. To further determine whether ErbB4 was correlated with these proteins, we characterized the changes in the levels of Bax (pro-apoptotic) and the colocalize with Bax. ErbB4 immunoreactivity colocalized with Bax in apoptotic pyramidal neurons of hippocampal fields. Quantitatively, about 80% of apoptotic pyramidal neurons in the CA2 fields expressed ErbB4 (Figs. 3A and 4A). Taken together, these results indicate that NRG1/ErbB4 signaling might serve as a survival signal in progressing AD.
Discussion
AD is characterized by progressive impairment of cognition and behavioural disturbance that strongly correlate with degeneration and death of neurons in the cerebral cortex and limbic brain areas, such as the hippocampus and the amygdala (Mattson 2004). Multiple lines of evidence demonstrate that the regulation of the Bcl-2 protein family is crucial for the maintenance of mitochondrial integrity and function thereby deciding a cell's fate after severe stress. The Aβ1-42 peptide induces cell death in human neuroblastoma cells as well as caspase-3 activation, initially via a Bax/Bcl-2 ratio increase (Clementi et al., 2006). Additionally, Bcl-2 and Bax induction has been shown to be related to hyperphosphorylation of tau and neuronal death induced by okadaic acid in the rat brain (Mattson & Meffert, 2006). Wide-spectrum gene expression studies have demonstrated similar depletion of the anti-apoptotic members of the Bcl-2 gene family in AD hippocampus and superior temporal lobe neocortex, and a shift in expression towards the more pro-apoptotic Bcl-2 family species Bax, Bad, Bid and Bik (Lukiw & Bazan, 2006). It is noteworthy that in this study, ErbB4 immunoreactivity colocalized with Bax in apoptotic pyramidal neurons of hippocampal fields. Despite this, the detailed mechanism involved remains to be clarified.
Recently, ErbB4 has been shown to play a key role in activity-dependent maturation and plasticity of excitatory synaptic function (Li et al., 2007a). Further, NRG1 has been demonstrated to activate ErbB4 and regulate GABAergic transmission in adult brains (Woo et al., 2007). Moreover, it was shown that NRG1 and ErbB4 immunoreactivity are associated with neuritic plaques in AD brains as well as in a transgenic model of AD (Chaudhury et al., 2003). These observations suggest that NRG1 may play a role in synaptic plasticity, maintenance, or regulation of synaptic structure, or some combination thereof in the adult brain.
In the present study, ErbB4 immunoreactivity was found to be significantly increased in the AD human brains and colocalized with Bax in apoptotic pyramidal neurons of hippocampal regions. However, whether increased ErbB4 immunoreactivity or ErbB4 colocalization with Bax in apoptotic pyramidal neurons were involved in survival or death pathways was not demonstrated. Regardless, our in vitro AD experimental results clearly showed that ErbB4 is necessary for NRG1 protection of neuronal cell death.
Similar to APP, ErbB4 is a substrate for γ-secretase and as such, represents the first cleavage by TACE to release a soluble extracellular peptide that contains the NRG1 binding site (ecto-ErbB4). The remaining membrane-anchored 80 kDa fragment (that is, ErbB4-CTF) is further cleaved in its transmembrane domain by presenilin-dependent γ-secretase to release the ErbB4 intracellular domain (ErbB4-ICD), which has been shown to translocate to the nucleus and to regulate transcription (Ni et al., 2001; Lee et al., 2002). In our data, we have also found that ErbB4 immunostaining was significantly increased in the nucleus, suggesting that the presenilin-dependent cleavage of ErbB4 may be involved in progressing of AD pathology. More work is needed to determine whether ErbB4-ICD has a physiological or pathological function in neurons. The results of the current study suggest that NRG1/ErbB4 signaling may be involved in AD neuropathology. Further investigation of the role of NRG1 in AD could be useful in understanding the pathogenesis of AD.
Collectively, our results clearly show that ErbB4 immunoreactivity was significantly increased in the AD brain, compared to age-matched controls and that ErbB4 immunoreactivity colocalized with Bax in apoptotic pyramidal neurons of hippocampal fields, suggesting that NRG1/ErbB4 signaling might serve as a survival signal in AD progression.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download