Introduction
Allergic asthma is a chronic obstructive inflammatory lung disease characterized by increased reactivity of airway smooth muscles and remodeling of airway tracts. Traditionally, asthma is thought to be a Th2-dependent disease and is known to be mediated by elevated serum IgE levels (Barrois et al., 2006). Th2 cytokines such as IL-4, IL-5, IL-9, and IL-13 play major roles in the initiation and perpetuation of the disease (Barrios et al., 2006; Tulic & Hamid, 2006; Doherty & Broide, 2007), although TNF-alpha, the Th1 cytokine, is also a critical factor in severe cases (Holgate et al., 2006). The prevalence of asthma is increasing over and over worldwide, and in addition, it is sometimes refractory to conventional treatment (Chanez et al., 2007).
Because it is well established that asthmatic patients suffer from oxidative stresses from abnormal airway inflammation (Kelly et al., 1999; Schock et al., 2003), a lot of studies have been devoted to elucidating the relationship between dietary intake and serum level of antioxidants and the prevalence and severity of the disease (Gao et al., 2008; Sackesen et al., 2008). One of the most probably associated dietary antioxidants with asthma is vitamin C (Harik-Khan et al., 2004).
Vitamin C is an essential micro-nutrient involved in several metabolic pathways (Naidu 2003) and is regarded as an important physiological antioxidant (Guaiquil et al., 2001). Most mammals produce vitamin C by themselves, while some mammals including men, some primates, and guinea pig do not. Thus, men have to be supplemented with exogenous vitamin C by taking fresh fruits and vegetables, and/or supplementary pills. It is well known that the deficit of vitamin C lead to the scurby (Naidu 2003).
Vitamin C is also known to modulate immune responses. Recently, it has been reported that mega-dose vitamin C administered in mice shifted immune responses toward Th1 with suppression of Th2 responses (Noh et al., 2003; Noh et al., 2005). Conversely, inadequate vitamin C supplementation in L-gulono-γ-lactone oxidase deficient mice which can not synthesize vitamin C by themselves (Maeda et al., 2000) resulted in a decreased Th1 response against H. pylri infection (Lee et al., 2008).
Considering the pathophysiology of asthma, vitamin C could be used for the treatment and/or management of asthma as an antioxidant and/or as a Th1-shifting agent. Actually, studies on the beneficial effects of high dose vitamin C intake in asthmatic patients have been performed by some authors. However, the effects are still in controversy (Fogarty et al., 2003; Fogarty et al., 2006; Tecklenburg et al., 2007; Kaur et al., 2009). This controversy might come from differences in experimental settings such as criteria for patient selection, recommended dosage of vitamin C, parameters measured, and so on.
In this study, we tried to determine the effect of vitamin C on asthma in an experimental animal model. Our results showed that vitamin C did not changed Th1/Th2 balance in asthma. However, it exerted some anti-inflammatory effects against lung inflammation.
Materials and Methods
Experimental animals
Eight week-old male Balb/c mice were purchased from BioLink (Seoul, Korea) and kept under 12 h dark and light cycle in the Animal Facility of our Institute. Food and water were given ad libitum. Animal experiments were permitted by the Institute of Laboratory Animal Resources Seoul National University (permission number SNU-060920-5), and all the animal experimental procedures were performed following the SOP of our Institute.
Induction of experimental asthma model
Mice were sensitized on 3 consecutive days by intraperitoneal
injection of 100 µg ovalbumin (OVA; Sigma, St. Louis, MO) immersed in a same volume of alum (Peirce, Rockford,
IL). On days 18, 19, 22, and 23, mice were challenged with intranasal dripping of 25 µg OVA/30 µl PBS. On days from 18 to 24, mice were given an intraperitoneal injection of either 3 or 5 mg vitamin C dissolved in 100 µl PBS or of a same volume of PBS. Each experimental group consisted of 8 mice. On day 24, plethysmography was done. All experiments were repeated 3 times.
Airway hyperresponsiveness (AHR)
AHR was assessed indirectly by measuring methacholine-induced airway obstruction. Mice were challenged with serially increasing concentrations of nebulized methacholine (Sigma, St. Louis, MO) in a range of 0~50 mg/ml for 3 min and were placed in a whole body plethysmography chamber (Allmedicus Co., Ltd, Model # OCP-3000) after each concentration of methacholine exposure. Breathing pattern was recorded for 2 min and enhanced pause (Penh) was calculated using a software program based on measured parameters such as expiratory time, relaxation time, peak expiratory flow, and peak inspiratory flow.
Bronchoalveolar lavage and tissue processing
Mice were sacrificed on day 25 by deep anesthesia. After anesthesia, blood was drawn from orbital plexus and serum was obtained after clotting in a refrigerator. Bronchoalveolar lavage fluid (BALF) and lung tissue were obtained immediately after sacrifice. BAL was done with 1 ml of saline. Obtained fluids were centrifuged (4,000 rpm, 10 min) to get sedimented cells and supernatants. Cells were resuspended in 300 µl PBS and were used for cell count and for monolayer cell spread by cytospin (Shandon Cytospin 2) (800 rpm, 10 min). Spread cells were stained with Wright`s staining solution (Sigma, St. Louis, MO) and used for differential cell counts. Supernatnats were kept frozen until use for cytokine ELISA (Biosource, Camarillo, CA).
After BAL, lungs were inflamed with 1 ml Bouin's solution and further fixed in the same solution for 24 h. Routine processes for paraffin embedding were carried out, and 6 µm-thick paraffin sections were made. Tissue sections were subjected to hematoxylin and eosin staining for routine microscopic observation, and to periodic acid-Schiff's staining for goblet cell observation in the airway epithelium.
Histopathological scoring of lung tissues
The intensity of peribronchial and perivascular inflammatory cell infiltration was scored as the approximate number of layers of cells around vessels or bronchiols. In a tissue section, the intensity of infiltration varied for each vessel and airway, and thus the most severely infiltrated one was regarded as a representative one for the section. The degree of goblet cell hyperplasia was evaluated as the ratio of respiratory epithelium composed of PAS-positive goblet cells to the total epithelium. To get the ratio, the length of all the respiratory tracts shown in the section was measured and summed with respect to normal or PAS positive area. Measurement was done using image analysis software RHINOCEROS 4.0 program.
ELISA for serum antigen-specific antibody titration
Titers of OVA-specific antibodies in the serum were determined as follows. Ninety six-well plates (Nunc, Rochester, NY) were coated with 100 µl of 3 µg/ml OVA/well at 4℃ overnight. Plates were then briefly washed and blocked with 1% skim milk in PBS for 1 h at room temperature. One hundred-fold diluted serum samples were applied to the first row of a plate and 4-fold serial dilution was done. All samples were duplicated. After 2 hour-incubation at room temperature, plates were washed with a PBS-0.05% Tween 20 mixture, and alkaline phosphatase-conjugated antibodies for each isotype were added. After incubation for 60 min at room temperature, plates were washed and p-nitrophenyl phosphate substrate (Amresco, Solon, OH) solution was added. OD values were measured at 405 nm. The antibodies used were as follows; goat anti-mouse polyvalent immunoglobulins (1 : 1,000 diluted, Sigma), goat anti-mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgE antibodies (1 : 1,000 diluted, Southern Biotech, Birmingham, AL). OVA-specific anti-sera obtained from previous experiments were collected in a tube and used as a standard serum in every ELISA plate. Titers were expressed as a relative value to the standard serum value.
Results
Vitamin C administered during challenge reduced airway resistance induced by methacholine
Airway resistance induced by methacholine inhalation was evaluated by plethysmography (Fig. 1). Airway resistances in PBS-injected asthma group were significantly higher with all concentrations of methacholine (P<0.05) compared to those of normal control group. In contrast, vitamin C-treated asthma group showed similar resistances to those of normal control group with 6.25, 12.5, 25 mg/ml of methacholine. However, resistance with 50 mg/ml of methacholine was elevated compared to normal control group, which was still lower than that of PBS-injected asthma group. These results indicate that vitamin C administered during antigen challenge reduced AHR.
Vitamin C treatment reduced the number of inflammatory cells in bronchoalveolar lavage fluid
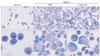
To approximate the extent of inflammation in each group, total numbers of inflammatory cells in bronchoalveolar lavage fluid (BALF) were counted. The numbers were standardized to the volume of fluid re-collected and depicted in Fig. 2 (left panel). In normal control group, BALF cells were scanty, while cell numbers substantially increased both in PBS-injected and vitamin C-treated asthma groups. Of note is that the number of cells from vitamin C-treated group was lower than that from PBS-injected group with statistical significance (P<0.05). To investigate the composition of BALF cells, they were spread on a glass slide, stained with Wright's solution, and the numbers of each immune cell including neutrophils, eosinophils, lymphocytes, and macrophage were differentially counted under light microscope. Majority of the cells from normal mice were macrophages (Fig. 3, left panels). In experimental groups, BALF cells were mainly composed of macrophages and eosinophils (middle and right panels in Fig. 3), which comprised 33.6% and 63.1%, and 20.2% and 65.4% of total cells in PBS-treated and vitamin C-treated groups respectively (Fig. 2, right panel). Occasional lymphocytes and neutrophils were also observed with a low frequency (2.9% and 0.4% in PBS-treated group, 3.7% and 0.7% in vitamin C-treated group). This compositional difference of cells between these two groups was statistically insignificant (P>0.05). Thus, vitamin C reduced total cell numbers in BALF, however, it did not affect the ratio of immune cells in BALF. Another finding that was observed was that macrophages from asthmatic mice revealed an activated appearance with a euchromatic nucleus, enlarged cell size, and cytoplasmic phagocytic vacuoles, while those from normal control mice showed quiescent appearance (lower panels in Fig. 3).
Vitamin C lowered the intensity of inflammatory cell infiltration around vessels and airways
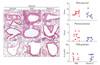
One of the histological hallmarks of lung inflammation accompanying asthma is cellular infiltration around blood vessels and airway tracts (Duan et al., 2004). To evaluate whether vitamin C treatment during asthma induction affected perivascular and/or peribronchiolar cell infiltration, histological examination was performed. Lung tissues from normal mice showed no perivascular nor peribronchiolar inflammatory cell infiltration at all (Figs. 4A and D). Meanwhile, asthma induction resulted in cell infiltration around blood vessels and airway tracts. The cell infiltration was more prominent around blood vessels than around airways. The thickness of cell infiltration was semiquantitatively evaluated by counting cell layers around the vessels or airways. As results, PBS-injected group showed perivascular infiltration of 6.4±1.5 cell layer-thick in average, which was thicker than that for vitamin C-treated group (5.0±2.9). This difference was statistically significant (P<0.05). The intensity of peribronchiloar cell infiltration also showed a tendency of reduction in vitamin C-treated group compared to PBS-injected group with average values of 1.2±0.9 and 2.6±1.8, respectively (P=0.058).
One of the characteristic changes following asthma induction is mucus hyperplasia of respiratory epithelium (Izuhara et al., 2009). Thus, we evaluated whether vitamin C treatment affected mucus hyperplasia. Lung tissues were PAS-stained and the degree of mucus hyperplasia was evaluated as the ratio of mucus-containing PAS-positive goblet cells per total respiratory epithelium (Figs. 4G~I). In normal mice, PAS-positive cells were hardly observed in the airway epithelium from trachea to terminal bronchiole (Fig. 4G). In experimental animals, epithelia of airways, especially those of large ones, were covered by PAS-positive mucus secreting cells. In PBS-injected mice, goblet cells covered 25.7±15.3% of all the respiratory epithelia in average, which was at a similar level to that for vitamin C-treated group (28.5±14.8%). Thus, it looks like that vitamin C given at the time of antigen challenge did not affect mucus hyperplasia.
Vitamin C did not shift immune responses with regard to Th1/Th2
Ovalbumin-specific serum antibody titers for each isotype were measured (Fig. 5) to determine whether vitamin C shifted immune responses toward Th1 in asthma animal model. Results showed that OVA-specific antibody titers were not different in the two experimental groups in both Th1-dependent isotypes (IgG1 and IgE) and a Th2-dependent isotype (IgG2a). In addition, when the secretion of Th1 cytokines such as IL-12, IFN-γ, and TNF-α and Th2 cytokines such as IL-4 and IL-5 in the BAL fluid were measured, they did not show any differences in both groups (data not shown).
Discussion
The aim of this study was to determine whether vitamin C administration did a beneficial role in animal asthma model. As results, vitamin C administered at a high dose at the time of antigen challenge reduced airway resistance provoked by methacholine. In addition, vitamin C reduced not only the number of inflammatory cells in BALF, but perivascular and peribronchilar inflammatory cell infiltration. All these results together suggest that vitamin C attenuated lung inflammation induced by asthma.
Asthma has been regarded as a Th2-dependent disease, and Th2 cytokines such as IL-4, IL-5, and IL-13 with TGFβ predispose and maintain this disease (Tagaya & Tamaoki, 2007; Broide 2008). Because a mega-dose vitamin C administered into the body shifts immune responses towards Th1 (Noh et al., 2003; Noh et al., 2005), we expected vitamin C administration at the time of antigen challenge in asthma model would attenuate the disease by shifting immune response toward Th1. Actually, this idea has been mentioned by some authors. A high dose vitamin C administration was recently reported to decrease eosinophilic infiltration in ovalbumin-induced asthma mouse model (Chang et al., 2009) and to decrease inflammatory cells in BALF, not only eosinophils but also neutrophils and lympocytes in asthmatic guinea pig model (Haines et al., 2010). In line with this concept, treatment using anti-cytokine agents or bacterial components aiming at the change of Th1/Th2 balance have been tired in moderate and severe asthmatics (Bjermer & Diamant 2004; Desai & Brightling, 2009). However, against the expectation, the results obtained in this experiment do not support Th1-shifting effects of vitamin C in asthma animal model. Vitamin C treatment did not shifted antigen-specific serum antibody isotype toward Th1 profile, neither changed cytokine profiles in BALF with respect to Th1/Th2 responses. In addition, cells in BALF from vitamin C-treated mice were composed mainly of macrophages and eosinophils just like in PBS-injected group. If Th1 shifting had been occurred by vitamin C administration, neutrophils were expected to prevail (Cohn et al., 1998). Vitamin C given at the time of sensitization did not affected Th1/Th2 balance either. When we treated mice with vitamin C for 10 days during which mice were intraperitoneally sensitized with OVA, it exerted absolutely no effects on asthma including Penh, BALF cell number, and lung inflammation (unpublished data). These results are against what previously reported with delayed type hypersensitivity reaction induced by keyhole lympet hemocyanin (Noh et al., 2005). In that experiment, administered mega-dose vitamin C clearly shifted immune responses toward Th1, nearly completely suppressing serum antigen-specific IgG1 and IgE antibody titers and rendering splenic T cells to secrete more IL-4 and less IFN-γ. We have no data to explain what made this discrepancy at this time. However, it has been reported that the immune response in animal asthma model varied depending on the route and frequency of antigen administration (Nelde et al., 2001). Another possible clue to explain the discrepancy is that dendritic cells, which play an initial and principal role in Th1/Th2 shaping, have many subtypes which lodge differently from location to location in the body. These subtypes of dendritic cells respond differentially to a same antigen in different ways (Kirby et al., 2001; Marleau et al., 2008). That might direct immune responses against a same antigen differently according to the route through which antigens enter the body. This point need to be further elucidated.
Even though no evidence of Th1 shifting was observed in this experiment, it is still true that the administered vitamin C attenuated the intensity of lung inflammation as evidenced by decreased cell numbers in BALF and less severe perivascular and peribronchial cell infiltration. These effects seems to be attributed to anti-inflammatory effects of vitamin C. Serum concentration of vitamin C has been supposed to be associated with inflammation in several epidemiological studies (Langlois et al., 2001; Wannamethee et al., 2006; Rizzo et al., 2008). In addition, anti-inflammatory effect of vitamin C has been clinically applied to pancreatitis (Du et al., 2003) with modest attenuation of serum inflammatory markers. These anti-inflammatory effects of vitamin C were ascribed to the anti-oxidant property of it. Or, some investigators insisted that vitamin C directly inhibited IkB kinase phosphorylation leading to eventual inhibition of NF-kB activation, which plays a critical role in inflammation (Cárcamo et al., 2002).
However, this anti-inflammatory effect has been denied by some authors (Scott et al., 2005; Hernandez et al., 2009; Kamgar et al., 2009). Thus, there are some controversies whether vitamin C is beneficial to inflammation. Our results support anti-inflammatory effects of vitamin C. That is, vitamin C given at the time of antigen challenge attenuated AHR and lung inflammation. However, the effect was not so much distinguished. Asthma is in nature a chronic inflammatory disease characterized by a phenominon known as "airway remodelling", which is contributed by subepithelial basement membrane thickening and fibrosis, increased smooth muscle cells, hyperplasia of mucus-secreting goblet cells, and so on (Hamid & Tulic, 2009). These changes takes time to occur and animal model of asthma usually does not reflect these changes satisfactorily because the disease model is not a chronic one (McMillan & Lloyd, 2004). Our model was also an acute one, and it should be necessary to evaluate the effects of vitamin C on the remodelling of airways in an established chronic type of asthma model (Zhou et al., 2008).
In conclusion, even though vitamin C did not show any Th1/Th2 shifting effects in this experiment, it still exerted moderate anti-inflammatory effects. Furthermore, we can not still rule out that chronic usage of vitamin C would exert more beneficial effects on asthma. Considering other beneficial effects and that vitamin C is not an expensive one, we can regard mega-dose usage of vitamin C as a potential supplementary modality for the management of asthma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download