Abstract
During the prostate cancer (PCa) development and its progression into hormone independency, androgen receptor (AR) signals play a central role by triggering the regulation of target genes, including prostate-specific antigen. However, the regulation of these AR-mediated target genes is not fully understood. We have previously demonstrated a unique role of HOXB13 homeodomain protein as an AR repressor. Expression of HOXB13 was highly restricted to the prostate and its suppression dramatically increased hormone-activated AR transactivation, suggesting that prostate-specific HOXB13 was a highly potent transcriptional regulator. In this report, we demonstrated the action mechanism of HOXB13 as an AR repressor. HOXB13 suppressed androgen-stimulated AR activity by interacting with AR. HOXB13 did neither bind to AR responsive elements nor disturb nuclear translocation of AR in response to androgen. In PCa specimen, we also observed mutual expression pattern of HOXB13 and AR. These results suggest that HOXB13 not only serve as a DNA-bound transcription factor but play an important role as an AR-interacting repressor to modulate hormone-activated androgen receptor signals. Further extensive studies will uncover a novel mechanism for regulating AR-signaling pathway to lead to expose new role of HOXB13 as a non-DNA-binding transcriptional repressor.
Androgen receptor (AR) has a key regulatory function in the growth and differentiation of normal and cancerous prostate epithelial cells by triggering the regulation of target genes such as jun, fos, myc, cdks, and fibroblast growth factor. Transactivation of AR is initiated upon binding to androgen. AR then translocates into the nucleus and binds as a homodimer to the cognate DNA response elements on the promoter, where it interacts with other transcriptional factors and activates downstream gene transcription. AR has distinct functional domains, a highly conserved DNA binding domain comprising two zinc finger motifs, a C-terminal ligand binding domain, and a poorly conserved amino-terminal domain that may contain transcriptional activation domains. AR can enhance or inhibit transcription by recruiting many co-regulators to the hormone-receptor complex. Moreover, it is believed that AR-mediated pathway plays a central role during androgen-independent progression of prostate cancer (PCa) cells. However, the regulation of these AR-mediated target genes under both hormone-dependent and -free environment is not fully understood.
Hox homeobox genes is generally considered as transcription factors that function during development to regulate axial regional specification during embryonic development and are expressed with temporal and spatial colineality. Despite intensive research, the mechanism of action of Hox proteins still remains unclear. Due to the presence of the DNA-binding homeodomain, a paradigm that the Hox proteins function as transcription factors was quickly established (Levine & Hoey, 1988). However, large numbers of Hox proteins alone do not bind to target DNA with high affinity or specificity (Shen et al., 1996), but require other cofactors to form cooperative DNA binding complexes, such as Pbx/Exd and /Meis/Prep/Htx (Mann & Chan, 1996). Pbx proteins physically interact with the YPWM motif of the Hox proteins, increasing their DNA binding affinity and defining their sequence specificity. However, Abd-B subfamily Hox proteins, including HOXB13, do not have a typical YPWM motif and consequently do not interact with Pbx/Exd. A few HOX target genes were described without cooperative DNA binding with partner proteins (Chariot et al., 1999; Raman et al., 2000a; Raman et al., 2000b). These proteins, comprising paralog groups 9-13, bind to DNA in the absence of cofactor proteins (Shen et al., 1997). The mechanism of transcriptional regulation by Abd-B hox proteins remains poorly understood.
HOX proteins are also involved in the regulation of coactivator function without DNA binding. Shen et al. reported that most HOX proteins, including the HOX-13 paralog, bind to CBP/p300 coactivators through their homeodomain (Shen et al., 2001; Shen et al., 2004). CBP/p300 does not form DNA binding complexes with the HOX proteins but instead prevents their binding to DNA, suppressing the HOX proteins' transactivating function. Conversely, HOX proteins inhibit the activity of CBP, suggesting that HOX proteins may inhibit CBP histone acetyltransferase activity and thus function as repressors of gene transcription. These lines of evidence suggest that HOX proteins function through a variety of pathways.
Due to the highly prostate-specific and AR-correlated expression pattern of HOXB13, we extensively studied the role of HOXB13 homeodomain protein in the regulation of AR-mediated signaling pathway (Jung et al., 2004a and b). Briefly, HOXB13 significantly suppressed androgen-activated AR transcriptional activity in a dose responsive manner while HOXB13 generally promoted the activity of other promoters, including RSV, SV40, and hormone-activated estrogen receptor. HOXB13 further down-regulated the expression of AR target proteins, including prostate specific antigen (PSA) and physically interacted with exogenous AR. Suppression of endogenous HOXB13 greatly promoted hormone-activated AR activity (up to 3 fold), suggesting that low level endogenous HOXB13 is very potent in the regulation of AR activity. Consequently, HOXB13 suppresses the growth of AR-expressing PCa cells, which can be counteracted by the addition of hormone-activated AR. These results suggest that HOXB13 functions as an AR modulator by specifically regulating this powerful growth signals. Since HOXB13 expression seems to be restricted in AR-expressing prostate cells in that study, balance of HOXB13 and AR may be important for cells to avoid abnormal proliferation. At the same time, loss of HOXB13 may be required for androgen-independent PCa cells to survive.
The objective of this report is to investigate the mechanistic involvement of HOXB13 in the regulation of AR-signaling. We tested to determine whether HOXB13 requires DNA-bound AR to inhibit AR activity. We also tested whether HOXB13 physically interacted with AR and this type of association resulted in disturbance of nuclear translocation of AR.
The pFLAG-HOXB13, pAd-GFP-HOXB13, and pGL-ARE4-Luc have been previously described (Jung et al., 2004a). The pPSA-luc contains the entire PSA promoter as previously described (Lee et al., 2002). Anti-AR antibodies were from Santa Cruz Biotechnology. Anti-HOXB13 antibodies were custom-made as previously described (Kim et al., 2010). Synthetic testosterone, R1881, was from NEN Life Science and used at a final concentration of 10 nM. Charcoal dextran-treated (CDT) fetal bovine serum (FBS) was from Invitrogen.
Human prostate cancer cell LNCaP was routinely cultured in RPMI media (Invitrogen) supplemented with 5% FBS at 37℃ in an atmosphere containing 5% CO2. All cultures were fed with fresh medium every 3-4 days.
Approximately 1×105 cells were plated in a 24-well plate 16 hours before transfection. To see the hormone effect, cells were grown under 5% CDT-FBS for three days before the transfection. The transfections were carried out using the Lipofectamine 2000 (Invitrogen) with 0.1 µg of reporter, 0.1 µg of test plasmid, and 2 ng renilla as described by the manufacturer's protocol. Six hours after transfection, the cells were washed and fed with medium containing 5% CDT-FBS. The cells were treated with either R1881 synthetic androgen or ethanol. After 36 hours, the cells were washed with PBS, lysed with 100 µl of passive lysis buffer, and assayed for luciferase activity as relative light units using the Dual Luciferase assay system (Promega). The transfection experiments were performed in triplicate and the results are reported as the mean ± S.D. The relative luciferase activity (RLU) was measured. All statistical tests were two-sided, and P values less than .01 were considered to be statistically significant.
LNCaP cells were infected with either Ad-GFP or Ad-GFP-HOXB13 at a multiplicity of infection (MOI) of 2 under the influence of androgen. Infectivity and cell viability were carefully monitored by fluorescence microscopy for 36 hours. Total RNA was purified using Qiagen RNeasy system. Quality control of RNA was made by both spectrophotometry and agarose gel separation of ribosomal RNA. Gene chip analysis was done on contractual basis with Indiana University Center for Medical Genomics (Indinapolis, Indiana, USA). Affymatrix GeneChip® Human Genome U133 was used and hybridization were performed in triplicate for each sample. Arrays were then scanned using scanner, controlled by Affymetrix GCOS software. Images were examined for defects. The Affymetrix® Microarray Suite version 5.2 (MAS5) algorithm analyzed the hybridization intensity data from GeneChip® expression probe arrays and calculated a set of metrics that described probe set performance. The average intensity on each array was normalized by global scaling to a target intensity of 1000.
Oligos (Integrated DNA Technologies) were annealed by heating up to 95℃ for 10 min and slowly cooling down to room temperature in TEN buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.1 M NaCl). An androgen response element (ARE) was derived from MMTV and double-stranded oligonucleotides, tgtacaggatgttct, were end-labeled with (32P-γ-) ATP using T4 polynucleotide kinase (New England Biolabs, Beverly, MA). Either nuclear extracts (2 µg) or GST-purified proteins (1 or 5 µg) were prepared as previously described. Labeled probe (10,000 cpm) and proteins were incubated with binding buffer containing 20 mM HEPES (pH 7.6), 10 mM (NH4)2SO4NaCl, 1 mM EDTA, 1 mM dithiothreitol, 30 mM KCl and 3 µg poly(dI-dC) (Sigma) at 25℃ for 30 min. Samples were subjected to electrophoresis at room temperate using 6% nondenaturing polyacrylamide gel in 0.5% TBE at 32 mA for 4 h. For competition experiments, 800X more unlabeled oligos were incubated with nuclear extracts for 30 min at 25℃ prior to the addition of the labeled probe.
LNCaP cells were grown under 5% CDT-FBS for 3 days. A 10 nM final concentration of R1881 or ethanol was added. Nuclear extracts were collected as previously described (Lee et al., 2003). Nuclear extracts (100 µg) were preincubated with 4 µg of anti-HOXB13 antibodies for 3 hours at 4℃, followed by adding protein A/G agarose (Santa Cruz Biotechnology). Immunoprecipitates obtained by centrifugation were washed with tris-buffered saline three times and eluted with 2X SDS loading buffer. Proteins were separated on 10% SDS-PAGE using the Novex electroporation system (Invitrogen). After proteins were transferred to PVDF membrane, immunoblotting was followed by using anti-AR antibodies primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Blots were developed by the ECL detection system (Pierce).
LNCaP cells were plated onto 4-well chamber slides under 5% CDT-FBS for 72 hours. Then, cells were given by either R1881 at 10 nM final concentration or ethanol. Cells were briefly risensd by PBS and fixed with 2% paraformaldehyde at room temperature for 10 minutes. Cells were preincubated with 3% normal goat serum followed by incubation with both anti-HOXB13 and anti-AR antibodies. After rinsing with PBS, cells were given by mixture of Alexa Fluor® 488 anti-mouse and 568 anti-rabbit antibodies (Invitrogen). After washing, slides were covered with anti-fade mounting agent and monitored with Zeiss LSM510 confocal microscopy.
The tissues were deparaffinized followed by microwave antigen retrieval in citrate buffer. Endogenous peroxidase activity was destroyed by treating tissue sections with 0.3% H2O2 followed by avidine-biotin blocking. After nonspecific reactivity was sequentially blocked by an avidin-biotin blocking reagent and 10% normal serum, the tissues were incubated with antibodies against HOXB13, AR, PSA, or transcription factor IID. Tissues were then incubated with appropriate second antibodies conjugated with biotin.
Then, the signals were amplified by the horseradish peroxidase-DAB detection method.
We have previously observed that HOXB13 exerted suppressive role in androgen-stimulated AR-mediated transactivation (Jung et al., 2004b). We first studied if there was any functional association between HOXB13 and AR using PSA promoter encompassing 6.1 kb of PSA promoter and enhancer region in LNCaP PCa cells. Transient transfection combined with reporter transcription analysis was performed. Using pGL-PSA-luc, HOXB13 repressed about 50% of androgen (R1881)-activated AR activity of PSA promoter while there was AR-stimulatory role in androgen-independent activation of PSA (Fig. 1A). Using four copies of androgen response elements cloned into a pGL-TATA-luc vector, pGL-ARE4-luc, transfection of HOXB13 dramatically inhibited androgen-activated AR transcriptional activity (Fig. 1B).
In order to identify the profiles of androgen-regulated genes affected by HOXB13, LNCaP cells were grown in charcoal dextran-treated (CDT)-FBS condition for 3 days. Either adenoviral recombinant HOXB13 or control virus was given to the cells followed by the addition of R1881 synthetic androgen for 36 hours. Purified RNA was analyzed by Gene Chip analysis. Out of 401 known and unknown potential HOXB13 target genes, there were 26 androgen-stimulatory genes and 4 androgen-inhibitory genes (Table 1). All of androgen-regulated gene expressions were counteracted by HOXB13. HOXB13 regulated the expression of most AR responsive genes, including NKX3.1, prostate-specific antigen, PDEF, and serpinI1.
Since HOX proteins are generally known as transcription factors with intrinsic DNA-binding ability due to the presence of a highly conserved homeodomain, we tested whether HOXB13 binds to the androgen responsive element (ARE) to modulate AR transcriptional activity. A gel mobility assay was employed. AR binding to ARE was used as a positive control due to the lack of information on HOXB13-binding DNA sequences. As shown in Fig. 2A, endogenous AR from LNCaP cells binds to 32P-labeled ARE (lane 1), whose signal was abolished by the addition of unlabeled ARE (lane 2). However, GST-HOXB13 (1~5 µg) did not bind to ARE (lanes 4-5). We then studied the interaction between HOXB13 and AR by co-immunoprecipitation assay. LNCaP cells were grown in the presence or absence of androgen. Nuclear extracts from cells were immunoprecipitated with anti-HOXB13 antibodies followed by Western blot analysis. As shown in Fig. 2B, AR was detected by its antibodies, while no band was detected with immunoprecipitates using normal IgG. This physical interaction between HOXB13 and AR was occurred regardless of the presence of androgen. Since HOXB13 does not possess the LXXLL or FXXLF motif, common motifs in AR-interacting proteins, HOXB13-interacting domain of AR seems to be different from other steroid receptor coactivators. These results suggest that HOXB13-mediated suppression of AR activity is not due to the binding of HOXB13 to ARE, but by interaction with AR.
In order to demonstrate that HOXB13-mediated AR suppression is due to the prevention of nuclear translocation of AR, we performed immunofluorescence assay in LNCaP cells which were grown in the absence or presence of androgen (Fig. 3). In the absence of synthetic androgen R1881, both HOXB13 and AR are generally distributed in both cytoplasmic and nuclear compartments of cells. Upon stimulation of R1881, nuclear translocation of AR was observed while HOXB13's cellular distribution was unchanged. These results suggest that HOXB13 did not disturb the nuclear translocation of AR.
Expression of HOXB13 is exclusively abundant in prostate (Sreenath et al., 1999; Hood et al., 2004; Jung et al., 2004b; Takahashi et al., 2004). Interestingly, expression of HOXB13 was limited to AR-expressing PCa cells and expression between HOXB13 and AR was not mutually regulated (Jung et al., 2004b). Due to the notorious heterogeneous and multifocal nature of PCa, we have localized expression of HOXB13 in various grades of prostate tumors using immunohistochemistry. As shown in Fig. 4, serial sections of benign prostate showed coexpressed phenomenon of HOXB13 and AR (upper row). High magnifications of some selected tumors cells were shown in bracket to demonstrate immunoreactive HOXB1. PSA expression was accordingly observed as an AR target protein. Transcription factor IID was used to show protein integrity. Most tumor cells coexpress HOXB13, AR, and PSA (middle row) while some tumor cells express neither HOXB13 nor AR with minor expression of PSA (bottom row). Note that HOXB13 was mostly localized in highly invasive tumor cells, leading technical difficulty to test molphologically identical field (middle row). Nevertheless, these results suggest that regulation of HOXB13 expression is tightly involved with the control of AR.
HOXB13 developmental protein predominantly expresses in the prostate but its biological role is not clear in this hormone-dependant organ. We have previously demonstrated that HOXB13 was an AR modulator, which ultimately inhibited AR-mediated growth signaling under the control of androgen. In this report, HOXB13-induced suppression of AR activity was mainly mediated through physical interaction with AR. HOXB13 did not bind to ARE to compete binding site with AR. In addition, HOXB13 did not interfere nuclear transloation of AR in the presence of androgen. Taken together, a model of HOXB13's role in the regulation of AR-mediated signaling is shown in Fig. 5. Upon stimulation of androgen, HOXB13 exert as an AR repressor by direct or indirect binding to AR. Sequestration of coactivators from AR by excessive HOXB13 prevent proper formation of AR coactivators complex, resulting in suppression of AR target gene expression. At the same time, HOXB13 can simply recruit more corepressors to the AR complex to show suppressive effect. Interestingly, interaction between HOXB13 and AR is made regardless of the presence of androgen, suggesting that HOXB13 have an important role in androgen-independent progression of PCa. In fact, we recently reported that HOXB13 was highly overexpressed in the majority of hormone refractory PCa compared to hormone responsive PCa (Kim et al., 2010). Overexpressed HOXB13 in these malignant PCa provides either a positive growth signal or better survivability in an androgen-deprived harsh environment.
Hox-13 paralog is especially important to the development of male accessory sexual organs, including the prostate (Podlasek et al., 1997; Podlasek et al., 1999a; Podlasek et al., 1999b). All Hox-13 genes except Hoxc13 are expressed in the prostate during embryonic development. However, Hoxa13 and Hoxc13 are no longer expressed after the embryonic stage (Podlasek et al., 1999a; Prins et al., 2001). Hoxd13 and/or Hoxa13-deficient mice showed morphological abnormalities in male accessory sex organs including the seminal vesicle and prostate (Podlasek et al., 1997; Warot et al., 1997; Podlasek et al., 1999a). Hoxc13, the last identified vertebrate Hox gene, has been shown to be limitedly expressed in the caudal extent of the spinal cord, tail bud and urogenital sinus in an androgen-independent manner (Zeltser et al., 1996; Sreenath et al., 1999). Recently, mice homozygous for Hoxb13 loss-of-function mutations showed overgrowth in all major structures derived from the tail bud (Economides et al., 2003) and malformation of ducts of the ventral prostate, including complete loss of secretory proteins (Economides & Capecchi, 2003). The phenotype shown in Hoxb13 mutant mice was similar to Nkx3.1 mutant mice and developed into swollen prostate in older mutant mice. Some isoforms of CD44 have been shown to be associated with tumor progression and metastasis including lung and gastric cancers (Lagorce-Pages et al., 1998; Wimmel et al., 2001). The mis-expression of CD44 in luminal epithelial cells observed in these mice is consistent with pre-neoplastic lesions in many tissue types. The involvement of Hoxb13 in ventral prostate may provide protection to this tissue from neoplasia in Nkx3.1 mutant mice since Nkx3.1 mutations do not cause prostatic intraepithelial neoplasias in the ventral prostate. However, there have been no studies on the biological function of HOXB13 in the tumorigenic process in human prostate. Due to its nature as a developmental gene showing a tissue-specific expression pattern, HOXB13 may be an important factor in aberrant prostate differentiation leading to tumorigenesis.
Our long term goal is to elucidate the function of HOXB13 and its action of mechanism during the prostate tumor development and progression into hormone-refractory tumors. We believe that elucidating the exact role/mechanism of HOXB13 in the regulation of AR activity is an important task to better understand a dogma in the process of PCa development. This report at least partly tested the hypothesis that HOXB13 is a unique repressor to modulate hormone-activated androgen receptor signals. This hypothesis is based on the following observations. First, unlike other AR cofactors, HOXB13 is exclusively expressed to the prostate and is a very potent AR repressor (Sreenath et al., 1999; Jung et al., 2004b; Takahashi et al., 2004; Edwards et al., 2005). Second, expression pattern of AR and HOXB13 is correlated, one's expression not being regulated by the other (Sreenath et al., 1999; Jung et al., 2004b). Third, despite general concept as a transcription factor, HOXB13 may have a function as a non-DNA binding protein being a transcriptional repressor (Zappavigna et al., 1994; Catron et al., 1995; Schnabel & Abate-Shen, 1996). Several HOX proteins, including HOXB13, prevent acetyltransferase function of CBP/p300 coactivators by physical interaction (Shen et al., 2001; Shen et al., 2004). Moreover, HOX proteins do not contain typical hormone receptor binding motifs (Schnabel & Abate-Shen, 1996; Zeltser et al., 1996). Experimental focus of this report was on regulatory mechanism of HOXB13 in AR signals while proposed hypothesis is not comprehensively tested yet. Further studies should include deletion and/or mutagenesis analyses to delineate interacting domains of HOXB13 and AR, elucidation of detailed association of HOXB13 with many nuclear receptor coactivators, and finally biological effects of minimal AR-interacting portion of HOXB13 through in vivo studies. Outcome of this long-term experiments will provide unique mechanism in androgen-mediated AR signaling pathway and HOXB13's clinical role in development of hormone refractory PCa.
Figures and Tables
Fig. 1
HOXB13 is an androgen receptor repressor in presence of androgen. (A) LNCaP cells were transiently transfected with 100 ng of pPSA-luc, 2 ng of renilla, and 100 ng of pFLAG-HOXB13 with or without 10 nM R1881, synthetic androgen. pFLAG-CMV was used as a counterpart of pFLAG-HOXB13. (B) similar to (A) except that pARE4-luc (100 ng) was used. Luciferase assays were performed 48 hours post-transfection. Values indicate as relative luciferase unit (RLU). Each bar represents the mean±S.D.

Fig. 2
HOXB13 physically interacts with androgen receptor (AR), not with DNA, to suppress androgen-stimulated AR activity. (A) To see if HOXB13's AR-suppressive function is due to DNA binding, electrophoretic mobility shift assay was performed. An androgen response element (ARE) was derived from MMTV and double-stranded oligonucleotides, tgtacaggatgttct, were labeled and used as a probe. First, LNCaP cells were grown in the presence of androgen followed by nuclear extraction. GST-HOXB13 protein was produced and purified. LNCaP nuclear extracts were used as a positive control (lane 1), whose signals were abolished by the addition of cold ARE (800X) (lane 2). Either GST (lane 3) or GST-HOXB13 (1-5 µg) (lanes 4-5) replaced nuclear extracts to see if HOXB13 binds to ARE. (B) For the in vivo interaction of HOXB13 and AR, co-immunoprecipitation was performed. LNCaP cells were grown under CDT-FBS for 3 days and treated with or without androgen and nuclear extracts were collected 48 hours after treatment. One hundred µg of proteins were mixed with 4 µg of anti-HOXB13 antibodies, followed by conjugation to agarose A/G beads. Normal IgG was used as negative control. Precipitated fractions (IP) were resolved by SDS-PAGE and analyzed by Western blot using anti-AR antibodies.
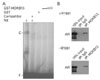
Fig. 3
HOXB13 does not disturb nuclear translocation of androgen receptor (AR) in the presence of androgen. LNCaP cells were grown under CDT-FBS for three days before stimulated by R1881 or vehicle for 6 hours. Cells were then fixed and co-immunostained for HOXB13 and AR and viewed by confocal microscopy. Nuclei were stained in blue using DAPI (a, e). AR (b, f) and HOXB13 (c, g) were stain in green and red, respectively. Merged images for AR and HOXB13 were shown in (d) and (h). The scale bars were 20 µm.
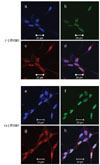
Fig. 4
Expression of HOXB13 and androgen receptor (AR) was mutually regulated in prostate tumors. Serial sections of prostate tissues were immunostained for HOXB13, AR, PSA, and TFID. Malignant tumors used in this study belong to combined Gleason score 9. While HOXB13 and AR were colocalized in some tumor cells, there also were HOXB13 and AR-negative tumor cells. Transcription factor IID (TFIID) was used as a positive control. Magnification, 10X.
References
1. Catron KM, Zhang H, Marshall SC, Inostroza JA, Wilson JM, Abate C. Transcriptional repression by Msx-1 does not require homeodomain DNA-binding sites. Mol Cell Biol. 1995. 15:861–871.
2. Chariot A, van Lint C, Chapelier M, Gielen J, Merville MP, Bours V. CBP and histone deacetylase inhibition enhance the transactivation potential of the HOXB7 homeodomain-containing protein. Oncogene. 1999. 18:4007–4014.
3. Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development. 2003. 130:2061–2069.
4. Economides KD, Zeltser L, Capecchi MR. Hoxb13 mutations cause overgrowth of caudal spinal cord and tail vertebrae. Dev Biol. 2003. 256:317–330.
5. Edwards S, Campbell C, Flohr P, et al. Expression analysis onto microarrays of randomly selected cDNA clones highlights HOXB13 as a marker of human prostate cancer. Br J Cancer. 2005. 92:376–381.
6. Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004. 306:640–643.
7. Jung C, Kim RS, Lee SJ, Wang C, Jeng MH. HOXB13 homeodomain protein suppresses the growth of prostate cancer cells by the negative regulation of T-cell factor 4. Cancer Res. 2004a. 64:3046–3051.
8. Jung C, Kim RS, Zhang HJ, Lee SJ, Jeng MH. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res. 2004b. 64:9185–9192.
9. Kim YR, Oh KJ, Park RY, et al. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol Cancer. 2010. 9:124.
10. Lagorce-Pages C, Paraf F, Dubois S, Belghiti J, Fléjou JF. Expression of CD44 in premalignant and malignant Barrett's oesophagus. Histopathology. 1998. 32:7–14.
11. Lee SJ, Kim HS, Yu R, et al. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mol Ther. 2002. 6:415–421.
12. Lee SJ, Lee K, Yang X, et al. NFATc1 with AP-3 site binding specificity mediates gene expression of prostate-specific-membrane-antigen. J Mol Biol. 2003. 330:749–760.
13. Levine M, Hoey T. Homeobox proteins as sequence-specific transcription factors. Cell. 1988. 55:537–540.
14. Mann RS, Chan SK. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996. 12:258–262.
15. Podlasek CA, Clemens JQ, Bushman W. Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J Urol. 1999a. 161:1655–1661.
16. Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev Dyn. 1997. 208:454–465.
17. Podlasek CA, Seo RM, Clemens JQ, Ma L, Maas RL, Bushman W. Hoxa-10 deficient male mice exhibit abnormal development of the accessory sex organs. Dev Dyn. 1999b. 214:1–12.
18. Prins GS, Birch L, Habermann H, et al. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev. 2001. 13:241–252.
19. Raman V, Martensen SA, Reisman D, et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000a. 405:974–978.
20. Raman V, Tamori A, Vali M, Zeller K, Korz D, Sukumar S. HOXA5 regulates expression of the progesterone receptor. J Biol Chem. 2000b. 275:26551–26555.
21. Schnabel CA, Abate-Shen C. Repression by HoxA7 is mediated by the homeodomain and the modulatory action of its N-terminal-arm residues. Mol Cell Biol. 1996. 16:2678–2688.
22. Shen W, Chrobak D, Krishnan K, Lawrence HJ, Largman C. HOXB6 protein is bound to CREB-binding protein and represses globin expression in a DNA binding-dependent, PBX interaction-independent process. J Biol Chem. 2004. 279:39895–39904.
23. Shen WF, Chang CP, Rozenfeld S, et al. Hox homeodomain proteins exhibit selective complex stabilities with Pbx and DNA. Nucleic Acids Res. 1996. 24:898–906.
24. Shen WF, Krishnan K, Lawrence HJ, Largman C. The HOX homeodomain proteins block CBP histone acetyltransferase activity. Mol Cell Biol. 2001. 21:7509–7522.
25. Shen WF, Montgomery JC, Rozenfeld S, et al. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol. 1997. 17:6448–6458.
26. Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999. 41:203–207.
27. Takahashi Y, Hamada J, Murakawa K, et al. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp Cell Res. 2004. 293:144–153.
28. Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dollé P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997. 124:4781–4791.
29. Wimmel A, Kogan E, Ramaswamy A, Schuermann M. Variant expression of CD44 in preneoplastic lesions of the lung. Cancer. 2001. 92:1231–1236.
30. Zappavigna V, Sartori D, Mavilio F. Specificity of HOX protein function depends on DNA-protein and protein-protein interactions, both mediated by the homeo domain. Genes Dev. 1994. 8:732–744.
31. Zeltser L, Desplan C, Heintz N. Hoxb-13: a new Hox gene in a distant region of the HOXB cluster maintains colinearity. Development. 1996. 122:2475–2484.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download