Introduction
Depending on the duration of the ischemia, permanent occlusion of the cerebral artery has been shown to result in characteristic pathophysiological events. In general, during focal cerebral ischemia, neuronal damage evolves over time and space and is not limited only to the lesion itself but is also observed in perilesional areas (i.e., the penumbra) (Witte et al., 2000).
The Na+-K+ adenosine triphosphatase (Na+/K+-ATPase) has been reported to be responsible for the energy dependent extrusion of Na+ and uptake of K+, and which represents an important component in the maintenance of ionic homeostasis both in astrocytes and neurons (D'Ambrosio et al., 2002). During cerebral ischemia, neuronal damage has been shown to be associated with an imbalance in ionic homeostasis, which is thought to be the result of decreased energy metabolism. This compromised energy supply to the cells results in the inhibition of the Na+/K+-ATPase and a runs-down in transmembrane Na+ and K+ ion gradients which are closely associated with the intracellular Na+ overloading and ischemic depolarization of neuronal cells (Fuller et al., 2003). There is general agreement that the ischemic depolarization during ischemia is probably due to the depression of Na+/K+-ATPase activity, and the resultant elevation of [K+]o and the interstitial accumulation of glutamate (Glu) from excitatory synaptic terminals (Ben-Ari 1990; Martin et al., 1994). It has been suggested that the Glu accumulation in the interstitial space results from the reverse operation of the Glu transporter by ischemia or anoxia may leads to cell death due to NMDA receptor-induced Ca2+ overload (Madl & Burgesser, 1993).
The Na+/Ca2+ exchanger 1 (NCX1) is a transmembrane protein that is not only expressed in the brain and heart but has also been found in many other tissues and cells, including kidney, skeletal muscle, smooth muscle, lung, and spleen (Quednau et al., 1997). NCX1 has been reported to catalyze the extrusion of one intracellular Ca2+ and the influx of three extracellular Na+ in each reaction cycle depending on the Na+ gradient generated by the Na+/K+-ATPase. It has been revealed that NCX1 can function in the forward and reverse direction, and that its activity is regulated by many factors including Na+, Ca2+, intracellular pH, and ATP (Boscia et al., 2006). In general, NCX1 plays a role in glial and neuron damage induced by ischemia, glucose deprivation, and excitotoxicity, although controversy remains as to whether net NCX1 activity is beneficial or detrimental (Matsuda et al., 2001; Pignataro et al., 2004). Recent studies demonstrated that inhibition of NCX1 by substitution of Na+ with Li+ and Cs+ affects NMDA-induced intracellular Ca2+ increase in glucose-deprived and depolarized cerebellar granule cells (Blaustein & Lederer, 1999; Kiedrowski 1999).
The N-methyl-D-aspartate (NMDA) type of ionotrophic glutamate receptor has been demonstrated to play a key role in neuronal plasticity, learning, and memory in the central nervous system due to its high Ca2+ permeability (Mori & Mishina, 1995). Although inappropriate activation of the NMDA receptor and neurotoxicity has been well described (Lipton & Rosenberg, 1994), little is known regarding the modulation of individual subunits that make up the NMDA receptors after ischemia. Recent studies demonstrated that treatment with the NMDA-antagonist MK-801 in ouabain, Na+/K+-ATPase inhibitor, -induced excitotoxicity attenuated the infracted volume of brain tissue exhibiting the ouabain-induced injury is indeed excitotoxic in nature which needs overestimation of glutamate receptors such as the NMDA receptors (Lees & Leong, 1996; Veldhuis et al., 2003). In particular, ouabain-induced drop in the driving force of Ca2+ influx via NMDA channels was offset by an increased driving force of reverse NCX1 (Czyz et al., 2002).
Structurally, NMDA receptors are hetero-oligomeric proteins formed by obligatory NMDA receptor 1 subunit (NR1) interacting with NMDA receptor 2A-2D subunits (NR2A-D), conferring functional variability (Monyer et al., 1992; Ishii et al., 1993). The prominent NR2 subunits in adult brain are reported to be NR2A and NR2B. Considerable interest has been placed on the potential involvement of NMDA receptors in the neurodegenerative process that follows ischemia or hypoxia. Given that glutamate receptors, and in particular the NMDA receptor subtype, allow an influx of extracellular Ca2+ after stimulation, changes in the properties or numbers of these receptors could lead to the presentation of inappropriate amounts of intracellular Ca2+ to the neurons (Besancon et al., 2008). Recent studies demonstrated that accumulation of glutaric acid (GA), analogue of glutamate, in the glutaric aciduria type I (MIM 231670) and chronic stimulation of NMDA simultaneously down-regulate the NR2B subunit and decreases Na+/K+-ATPase activity (Resink et al., 1996; Kölker et al., 2002). However, NR2B was up-regulated by tetrodotoxin (Audinat et al., 1994), suggesting a contribution of spontaneous electrical activity to block the fast Na+ current in the neuronal cells.
The present study therefore aimed at examining whether the protein expression of Na+/K+-ATPase, NCX1, and functional NMDA receptor subunits (NR2A and NR2B) in the ischemic penumbra were altered. This was done because neurons in the penumbra undergo acute and delayed elevations of intracellular Na+ and Ca2+ levels through the significant interactions and feedback between the glutamate-dependent NMDA receptors and Na+ and Ca2+ ion channels (Na+/K+-ATPase and NCX1), which have been reported to directly or indirectly, lead to cell death after focal cerebral ischemia (MacDonald et al., 2006; Besancon et al., 2008).
Materials and Methods
Induction of focal cerebral ischemia in rats
All studies were carried out in a 9-week-old male Sprague-Dawley rats (n=16, 250~280 g) that had free access to drinking water and standard rodent food pellets. The experimental procedures were reviewed and approved by the Animal Care and Use Committee of Dongguk University (IRB: 09-45). Further, animal care and use were in accordance with the guidelines of the National Institutes of Health (Bethesda, MD). Focal cerebral ischemia was induced by occlusion of the left middle cerebral artery as described previously (Hasegawa et al., 1994).
Anesthesia was induced with 3% isoflurane in a mixture of oxygen/nitrous oxide (30 : 70) and rats were maintained with 1% isoflurane in the oxygen/nitrous oxide gas mixture. A catheter was inserted and positioned in the femoral artery and arterial blood pressure was measured and recorded continuously throughout the procedures. Body temperature was monitored continuously during all procedures using a rectal thermometer probe. Temperature control was accomplished with the aid of a heating pad which was kept at 37℃.
Under the dissecting microscope, left middle cerebral artery was occluded for 3 h, 6 h, and 24 h using a 4-0 mono filament (3 cm in length) coated with a mixture of silicone resin. Sham-operated controls rats were subjected to middle cerebral artery surgery without occlusion. After 3 h, 6 h, and 24 h of occlusion of the middle cerebral artery, rats were anesthetized with isoflurane again and the brain tissues were removed for 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma Aldrich Corp., St Louis, MO) staining.
TTC staining for infarction and penumbra zones
Rats were sacrificed and their brains were quickly removed and sectioned into 2-mm-thick slices starting from the frontal pole using a Brain Matrix Slicer (Vibratome Co.) (n=4). Slices were then immersed in TTC in a Petri dish and incubated at 37℃ for 20 minutes. Slices were flipped at the 10-minute mark to ensure staining of anterior and posterior faces.
Cresyl violet staining
At the scheduled time, sham-operated (n=3) and ischemic rats (n=3) were reanesthetized and their brain were fixed with a transcardiac infusion of 4% paraformaldehyde following perfusion with isotonic saline to remove blood from the cerebral vasculature. The brain was removed and post-fixed in the same fixative for 12 hours. Perfused brains were then paraffin-embedded and serial coronal sections 5 µm thick were obtained at the level of dorsal third ventricle (bregma-4.16 mm). Paraffin wax was removed in xylene over night at room temperature (RT) and the sections were rehydrated with ethanol (99%, 96%, 70%). After washing in distilled water, the sections were then stained with cresyl violet for 30 minutes at RT. The sections were then treated successively with ethanol (50%, 70%, 95%, 100%) and a differentiator (glacial acetic acid and 95% ethanol).
SDS-PAGE and immunoblotting
Penumbral or control tissues were removed from the TTC-stained brains of ischemic (n=4) and sham-operated rats (n=3), respectively for immunoblotting analysis. For protein extraction, the tissue was homogenized in homogenizing buffer (0.32 mM sucrose, 25 mM imidazole, 1 mM ethylenediaminetetraacetate (EDTA), pH 7.2 containing 8.5 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride). Samples of homogenates were run on 9~15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad Mini Protean II) in duplicates in which one gel was run in parallel and subjected to Coomassie blue (Coomassie brilliant blue 0.3 g, 2-propanol 200 ml, acetic acid) staining to assure identical loading. The other gel was subjected to immunoblotting.
After electrophoresis, the separated protein was transferred to nitrocellulose membrane in a buffer solution containing 50 mM Tris-base, 380 mM glycine, and 20% methanol. Membranes were blocked with 5% milk in phosphate buffer solution-T (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 h and incubated overnight at 4℃ with rabbit anti-Na+/K+-ATPase affinity purified polyclonal antibody (Chemicon, Temecula CA, USA, 1: 1,000), rabbit anti-NCX1 affinity purified polyclonal antibody (Chemicon, Temecula CA, USA, 1 : 2,000), rabbit anti-NMDA receptor subunits (NR2A, NR2B) polyclonal antibody (gifted from Moon, 1 : 5,000), mouse anti-neuronal nuclei (NeuN) monoclonal antibody (Chemicon, Temecula CA, USA, 1 : 1,000), mouse anti-glial fibrillary acidic protein (GFAP) monoclonal antibody (Boehringer Mannheim Biochemica, Philadelphia, USA, 1 : 2,000), and mouse anti-2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase) monoclonal antibody (Chemicon, Temecula CA, USA, 1: 1,000). The sites of antibody-antigen reaction were visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies (P447 or P448, diluted 1 : 3.000; DAKO, Glostrup, Denmark), an enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech, Little Chalfont, UK) system and exposure to photographic film (Hyperfilm ECL, RPN3103K, Amersham Pharmacia Biotech, Little Chalfont, UK). The immunoblot signal developed by ECL system was quantified using Scion Image software (version 1.59).
Results
Infarction and penumbra after focal cerebral ischemia
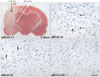
In the current study, experimental focal cerebral ischemia was induced in rats by the permanent middle cerebral artery occlusion for 3, 6, and 24 hours. Slices were divided into two zones, i.e., infarction zone (marked in black arrow) and penumbral zone (marked in white arrows) in the ipsilateral hemisphere according to the TTC staining pattern (Fig. 1A, pMCAO-3h). The penumbra was defined in static terms as the cellular interface between the infracted core cells that were committed to die and unaffected area of normal blood flow. The - numbers represent distances from the bregma. Cresyl violet staining was undertaken to examine whether the severity of neuronal injury in the penumbra was associated with duration of focal cerebral ischemia. Viable cells (arrows) were significantly decreased from 3 to 6 h after focal cerebral ischemia (Fig. 1B, C). Moreover, viable cells were not detected 24 h after focal cerebral ischemia which was very similar to that of the ischemic core (Fig. 1D). This finding indicated that the extent of neuronal damage was associated with the duration of focal cerebral ischemia.
Altered expression of Na+/K+-ATPase and NCX1 in the penumbra after focal cerebral ischemia
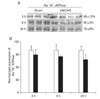
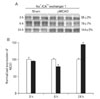
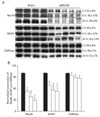
To evaluate the effect of focal cerebral ischemia in the penumbra, immunoblotting analyses of Na+/K+-ATPase were performed (Fig. 2A). The expression of Na+/K+-ATPase was not significantly altered as compared with that of the sham-operated controls at 3 or 6 h following focal cerebral ischemia. However, the expression of Na+/K+-ATPase was significantly decreased at 24 h of focal cerebral ischemia (78±6% of sham-operated controls, n=4, *P<0.05) (Fig. 2B). Furthermore, the expression of NCX1 was significantly increased 24 h after focal cerebral ischemia as compared with sham-operated controls (144±2% of sham-operated controls, n=4, *P<0.05) (Fig. 3A, B).
Altered expression of NR2A and NR2B in the penumbra after focal cerebral ischemia
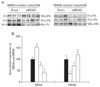
Three hours after focal cerebral ischemia, the expression of NR2A-containing NMDA receptor in the penumbra was significantly increased while the expression of NR2B-containing NMDA receptor was significantly decreased (NR2A: 153±9%; NR2B: 37±2%, n=4, respectively, *P<0.05) as compared with those of sham-operated controls. However, the expression of NR2A and NR2B in the penumbra was reversed 24 h after focal cerebral ischemia (NR2A: 40±7% of sham-operated controls; NR2B: 120±16% of sham-operated controls, respectively, n=4, *P<0.05) (Fig. 4A, B).
Altered expression of neural and astroglial cell proteins in the penumbra after focal cerebral ischemia
Immunoblotting analyses demonstrated that expression of neural (NeuN) and astroglial (GFAP) cell proteins was affected in the penumbra three to 24 h after focal cerebral ischemia (Fig. 5A). The expression of NeuN was significantly decreased and their expression was closely associated with the duration of ischemia (3 h: 52±6%; 6 h: 38±12%; 24 h: 29±8% of sham-operated controls, respectively, n=4, *P<0.05). The expression of GFAP was also directly affected by the duration of ischemia (3 h: 69±5%; 6 h: 56±12%; 24 h: 53±14% of sham-operated controls, respectively, n=4, *P<0.05) (Fig. 5B). In contrast, the expression level of CNPase for oligodendrocytes in the penumbra was unchanged following focal cerebral ischemia (Fig. 5A, B).
Discussion
The present study revealed that 1) the altered expression of Na+/K+-ATPase and NCX1 in the penumbra after focal cerebral ischemia indicates that deranged transport of Na+, K+, and Ca2+ which is closely associated with the NMDA receptor-mediated Ca2+ influx ; 2) the different expression of NR2A-containing- and NR2B-containing glutamate-dependent NMDA receptors in the penumbra may play different roles depending on the duration of ischemia; 3) prominent decrease of NeuN than that of GFAP in the penumbra may suggest that neurons in the penumbra are likely to be more susceptible to ischemic injury than astroglia.
Reduction of Na+/K+-ATPase in the penumbra indicates the disruption of intracellular Na+ and K+ homeostasis after focal cerebral ischemia
Middle cerebral artery occlusion produces regions of brain with near complete and incomplete ischemia (reduced blood flow). In general, areas of mild ischemic injury occur where Na+/K+ ATPase is preserved, while in areas of more severe ischemia, ATP levels are low and Na+/K+-ATPase activity is reduced (D'Ambrosio et al., 2002). Therefore, significant reduction of Na+/K+-ATPase in the penumbra at 24 h as compared with those of 3 and 6 h indicates a deleterious effect of 24 h of ischemia. Furthermore, the current study verified the histological findings demonstrating that the extent of neuronal damage depends on the duration of ischemia.
Several studies have examined the role of Na+/K+ ATPase ion channels in hypoxic-ischemic neuronal damage and have concluded that Na+ influx is an important initiating event leading to anoxic damage (Stys et al., 1992; Tasker et al., 1992). The decreased Na+/K+ ATPase expression in the present study postulates the disturbance of NCX1 and/or NMDA-induced Ca2+ influx by enhanced cellular K+ efflux and Na+ influx which result in ischemic depolarization in neurons and astrocytes. In general, Ca2+ influx or extrusion of NCX1 Depends on the ability of Na+/K+ ATPase to pump K+ and Depolarized the plasma membrane (Silver et al., 1997). The reduction of Na+/K+ ATPase on NMDA-induced Ca2+ influx also might be related to an enhancement of Ca2+ permeation of NMDA channels (Czyz et al., 2002). Furthermore, intracellular Na+ accumulation in astrocytes can contribute to glutamate release, which occurs by reversal of the Na+/glutamate cotransporter (Anderson & Swanson, 2000). This cotransporter normally mediates the entry of two Na+ ions along with one molecule of glutamate and represents an important mechanism of glutamate uptake by astrocytes, which ensures neuronal survival (Storck et al., 1992). If it is reversed due to excessive intracellular Na+, astrocytes begin to promote glutamate release and might contribute to neuronal damage during ischemia. Therefore, intracellular Na+ accumulation plays a critical role in NCX1 and/or NMDA-induced neuronal cell death by participating in mechanisms that brings about Ca2+ overload and accelerates glutamate release from the astrocytes.
In a normal brain, NCX1 is thought to be important in buffering neuronal intracellular Ca2+ by transporting one Ca2+ out of cells and three Na+ into the cells (Blaustein & Lederer, 1999). However, based on vitro and in vivo studies, it appears that during and following cerebral ischemia, it is likely that under depolarizing conditions, NCX1 can contribute to Ca2+ influx and neuronal injury. After NCX1 reverses, any further depolarization of the plasma membrane increases the electrochemical driving force of Ca2+ influx via this pathway (Hansen & Zeuthen, 1981; Benveniste et al., 1984). The role of reverse NCX1 in mediating toxic Ca2+ influx is supported by neuroprotective effects of NCX1 inhibitors (Schröder et al., 1999; Matsuda et al., 2001). The neurotoxic mechanism that leads to penumbra cell death in response to elevated intracellular Na+ in the current study represent the reverse operation of the plasma membrane NCX1, engaged by plasma membrane depolarization and intracellular Na+ overload through a decreased Na+/K+-ATPase. However, reverse NCX1 does not significantly contribute to Ca2+ influx after inactivation of NMDA receptors (Kiedrowski 2001). The mechanism of Na+-dependent Ca2+ influx requires open NMDA channels, because occlusion of the channels with MK-801 almost completely inhibited Ca2+ accumulation. Our results suggest that increased reverse NCX1 in the penumbra participates in Na+/K+-ATPase-dependent amplification of NMDA-induced Ca2+ influx in ischemic depolarized neuronal cells.
Altered expression of NR2A and NR2B, which depends on the duration of focal cerebral ischemia, could play variable roles in secondary brain cell injury in the penumbra
Our results indicate that increased NR2B in the penumbra after 24 h of focal cerebral ischemia may be comprised of combinations of NR2B subunit, along with the NR1 subunit (Monyer et al., 1992). Pharmacologic studies show that NR2B-containing NMDA receptor channels, expressed in Xenopus oocytes, exhibits a higher affinity for L-glutamate and considerably longer offset decay time courses following brief application of L-glutamate than the NR1-NR2A channel (Meguro et al., 1992). In particular, the offset decay time course is thought to be crucial for the determination of intracellular Ca2+ concentration (Perkel et al., 1993). These reports suggest that NR2B-containing NMDA receptors are more efficient than receptors containing NR2A in the process of Ca2+ influx. Chronic incubation with GA resulted in a down-regulation particularly of the NR2B subunit and reduced the NMDA receptor-mediated increase in intracellular Ca2+ (Kölker et al., 2002). In the present study, we demonstrated a reduction of Na+/K+ ATPase and increased expression of NCX1 and NR2B in the penumbra by focal cerebral ischemia. Because the Na+/K+ ATPase is particularly important to evoke the ischemic depolarization, decrease in Na+/K+ ATPase would result in a relief of the voltage-dependent Mg2+ block of NMDA receptors (Gegelashvili & Schousboe, 1997) and further increase of Na+-dependent Ca2+ influx mediated by NR2B-containing NMDA receptors and NCX1. In addition, extrasynaptic NR2B-containing NMDA receptors antagonize nuclear signaling to cAMP response element binding protein (CREB), block induction of brain derived neurotrophic factor (BDNF) expression, and are involved in mitochondrial dysfunction and cell death (Hardinghan et al., 2002).
Significant increase of NR2A 3 h after focal cerebral ischemia might couple with the compensatory response to diminish the Ca2+ which is associated with lower affinity for glutamate and considerably shorter offset decay time for Ca2+ compared with those of NR2B-containing NMDA receptors. This result suggests the possibility that the other Ca2+ ion channels beyond the NMDA receptors play a major role in the neuronal injury 3 h following focal cerebral ischemia. Moreover, depending on the type of NMDA receptor, Ca2+ entry can determine the biological outcome of Ca2+ signaling, as shown by site-specific differences in the regulation of CREB-mediated transcription (Hardinghan et al., 2002). Increases in the synaptic NMDA receptor subunit, NR2A, 3 h after focal cerebral ischemia may be associated with neuronal survival in the penumbra given that synaptic NMDA receptors promote nuclear signaling to CREB, induce BDNF gene expression, and activate an anti-apoptotic pathway (Rumbaugh & Vicini, 1999).
Differential expression of NeuN, GFAP, and CNPase in the penumbra depending on the duration of ischemia
There are many instances in which focal brain lesions also seem to have an impact on the function of surrounding or remote brain areas due to the fact that the brain can be considered as a network with multiple and intricate connections (Beck et al., 1996). Therefore, it is very important to analyze in detail which prognosis of focal cerebral ischemia is a direct consequence of the lesion, the perilesional area, or a reaction of the surrounding brain to the lesion. The most remarkable decrease of neural (NeuN) and astroglial protein (GFAP) 24 h after focal cerebral ischemia supports evidence demonstrating that increased NCX1 and NR2B-containing NMDA receptor is closely associated with Ca2+-dependent neuronal injury in the penumbra. Moreover, further declines of NeuN than that of GFAP in the penumbra may suggest that neurons in the penumbra are likely to be more susceptible to ischemic injury than astroglia. The underlying mechanisms for the susceptibility of neurons to ischemic insults may be explained in terms of glucose metabolism. Recent studies using multiphoton microscopy demonstrated that neurons use primarily oxidative metabolism, whereas astrocytes are glycolytic (Kasischke et al., 2004). In addition, glucose uptake in primary cultured astroglias was increased in response to the elevated extracellular K+ than that of neurons (Yu et al., 1989).
In conclusion, the current study suggest the new intracellular Ca2+ overloading mechanisms after focal cerebral ischemia in which Ca2+ influx through the reverse mode of NCX1, Intracelluler Ca2+ overhead is positively reinforced by glutamate-dependent NR2B-containing NMDA receptors which is triggered by deranged transport of Na+ through decreased Na+/K+-ATPase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download