Introduction
The mammalian brain consists of billions of neurons, including thousands of cell types that are connected into circuits by trillions of synapses. Detailed knowledge of neuronal connectivity patterns is indispensible for studies of various aspects of brain function. The wiring patterns found among various types of neurons via specific synaptic connections are the basis of the functional logic employed by the brain for information processing.
Dopaminergic neurons play a profound role in diverse brain functions, including movement, mood, attention, and visceral functions. In the ventral midbrain, dopamine is synthesized in the ventral tegmental area (VTA) and substantia nigra (SN) (Prakash & Wurst, 2006). The locus coeruleus (LC) is the most important noradrenaline-synthesizing cell group in the brain. This region provides widespread and divergent axonal innervation to all regions controlling behavioral and sensorimotor responses to external stimuli, including the cerebral cortex, striatum, hippocampus, thalamus, cerebellum, and the nuclei of the brainstem and spinal cord.
Conventional neuroanatomical tracing methods utilize axonal transport of tracers, such as horseradish peroxidase or wheat germ agglutinin (WGA), via neuronal microtubule systems, followed by detection of tracers using immunohistochemical methods (Köbbert et al., 2000). WGA is a plant lectin that has been used as a highly sensitive tracer for neuroanatomical and neuronal mapping studies (Gonatas et al., 1979; Broadwell & Balin, 1985; Fabian & Coulter, 1985; Itaya 1987). The WGA protein is a small 18-kD cysteine-rich lectin that binds to N-acetylglucosamine and sialic acid carbohydrate moieties found on glycoproteins and glycolipids. Because these WGA receptors are commonly expressed on the plasma membrane surface of most types of neurons in the central nervous system, WGA has been used as an effective tracer for a variety of neural pathways. WGA is efficiently taken up into neurons by endocytosis and is transported to axons and dendrites in both the anterograde and retrograde directions. Injection of WGA in well-mapped neural pathways has shown that labeling occurs in both first- and second-order neurons and their processes, indicating that WGA is trans-neuronally transported.
Selective labeling of small neuronal populations of a given phenotype is difficult using conventional methods because the tracers must be delivered by microinjection or local application. These delivery methods allow tracers to be taken up by all neurons at the injection site, resulting in nonspecific labeling of unrelated pathways. To overcome these problems, novel genetic approaches for visualization of specific trans-synaptic neuronal pathways have been developed that introduce a cDNA encoding a transsynaptic protein, such as WGA or the nontoxic tetanus toxin C-fragment (TTC), as a transgene under the control of cell-type specific promoter elements (Yoshihara et al., 1999; Oh et al., 2009). This genetic tracing method allows selective expression of tracers in specific neurons using cell-type specific promoters. Therefore, the selection of relevant cell-type specific promoters is critical for precise neuronal circuit mapping studies using this method. To date, the precise afferent and efferent connections of the VTA and LC have not been well characterized. Therefore, we analyzed the cell-type specificity of transgene expression under control of the TH promoter in the midbrain region.
Materials and Methods
Experimental animals
Twelve adult male Sprague-Dawley rats (280~300 g; OrientBio, Seongnam, Korea) were used in this study. Animal maintenance and treatment were carried out in accordance with the Principles of Laboratory Animal Care (NIH publication number 85-23, revised 1985) and the Animal Care and Use guidelines of Kyung Hee University, Seoul, South Korea. Animals were housed at an ambient temperature of 18~23° and at a relative humidity of 10~60% under a 12-h light-dark cycle. Animals were allowed free access to water and food.
Recombinant adenovirus construction
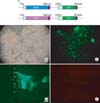
We used pAdeasy system for recombination of Adenovirus gene (He et al., 1998). A gene cassette containing the WGA or red fluorescent protein (RFP) cDNA under control of the TH promoter was inserted into the pAdTrack plasmid, followed by insertion into pAdEasy1 (Fig. 1A). Recombinant adenoviral DNA (10 µg) was digested with PacI and transfected into HEK293 human embryonic kidney cells in a 10-cm dish using Lipofectamine (Invitrogen, Carlsbad, CA). Viruses were harvested from transfected HEK293 cells 5 days after transfection and were amplified one time in HEK293 cells in ten 15-cm dishes. Viral particles were purified by cesium chloride density gradient ultracentrifugation and dialyzed against a viral storage solution (10 mM Tris, pH 7.4; 135 mM NaCl; 1 mM MgCl2; 5% sucrose). Viral stocks were titered using the end-point dilution method. Cell-type specificity of the TH promoter was then analyzed. Cell-type-specific expression of GFP and RFP was observed under a fluorescent microscope.
Stereotaxic injection of the adenoviral vector and brain section preparation
Adenovirus (1.5 µl of a 3×1011-EFU/ml solution) was unilaterally injected into the rat LC (AP: 9.8 mm relative to bregma, L: +1.3 mm from the midline, and DV: -6.5 mm below the dura), VTA (AP: -4.7 mm relative to bregma, L: +1.6 mm from the midline, and DV: -8.0 mm below the dura) or cerebellum (AP: -9.9 mm relative to bregma, L: +1.2 mm from the midline, and DV: -5.5 mm below the dura). We injected adenovirus on site of LC and VTA. But, we injected continuously through withdrawal of needle for cerebellum injection. Three days after stereotaxic injection, rats were anesthetized with pentobarbital sodium (60 mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4. Brains were isolated and post-fixed in the same fixative overnight. The brains were subsequently cryoprotected with 30% sucrose in 0.05M phosphate-buffered saline (PBS, pH 7.4) for 48 h and sectioned coronally into 40-µm sections for histological analyses.
Immunohistochemistry and immunofluorescence
After three washes with PBS each for 5 min, sections were processed for the immunohistochemical detection of WGA using goat polyclonal anti-WGA antibody (1 : 2,000; Vector, Burlingame, CA). Free-floating sections were incubated for 48 h in PBS (4℃) containing anti-WGA antibodies (1 : 2,000), 0.3% (v/v) Triton X-100, 0.05% (w/v) bovine serum albumin, and 1.5% (v/v) normal horse serum. Using the Vectastain-Elite kit (Vector, Burlingame, CA), we incubated sections with horse anti-goat secondary antibodies (1 : 100) for 90 min and with avidin-biotin-peroxidase complex (1 : 100) for 1 h at room temperature. The sections were reacted with 0.02% (w/v) 3,3'diaminobenzidine tetrahydrochloride and 0.01% (v/v) H2O2 for about 3 min. Following incubation, sections were washed three times in PBS, for a total wash time of 15 min. Sections were dehydrated with alcohol and xylene and mounted with Permount solution. Images were captured using an Axioscope and Axiocam CCD camera (Zeiss, Ober Kochen, Germany).
For immunofluorescence staining, brain sections were incubated with goat anti-WGA antibodies (1 : 2,000) at 4℃ for 12~15 h, then we washed sections with PBS for 15 min, followed by incubation with biotinylated horse anti-goat IgG (1 : 200; Vector) for 1.5 h. Sections were then incubated 1 h with streptavidin-rhodamine or strepatavidin-FITC (1 : 200; Vector). Hoechst 33342 incubation for 3 min was used to visualize nuclei. Slides were mounted using Vectashield mounting medium (Vector). Fluorescent images were captured using a confocal microscope (LSM 510-META, Zeiss).
Results
Cell type specific expression of transgene
A recombinant adenovirus (pAdeasy-TH-RFP-CMV-GFP) that expresses the RFP gene under control of the cell-type-specific TH promoter and the GFP gene under control of the general CMV promoter was tested in HEK293 cells. Five days after transfection with pAdeasy-TH-RFP-CMV-GFP, strong GFP expression was observed in HEK293 cells, but RFP was not expressed, consistent with expected results. We next injected the TH-RFP-CMV-GFP recombinant adenovirus into the cerebellum of adult male Sprague-Dawley rats. Three days after injection with the recombinant adenovirus, GFP was highly expressed in the cerebellar cortex. Adenovirus can infect all phenotype of cells, including neurons and glia. CMV promoter can induce GFP expression in all infected cells. Therefore, GFP positive cells are Prukinje cells, granule cells and glia. In contrast, RFP was not expressed in the injected side of the cerebellum (Fig. 1).
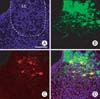
The TH-RFP-CMV-GFP recombinant adenovirus was next injected into the LC of adult rats. Three days after injection, strong GFP expression was observed in the injected side of the midbrain. In addition, RFP was also highly expressed in several neurons of the LC. The merged image shows that RFP colocalized with GFP in the same neurons (Fig. 2).
Tracer gene expression in specific brain regions
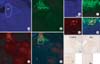
We also tested a cell-type-specific WGA-expressing recombinant adenovirus (TH-WGA-CMV-GFP) by injection into the VTA of male Sprague-Dawley rats. Three days after recombinant adenovirus injection, GFP was highly expressed at the site of injection. Furthermore, immunohistochemical staining for WGA demonstrated the presence of WGA-immunoreactive cells in regions adjacent to the injected site (Fig. 3).
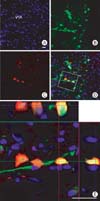
The WGA-expressing recombinant adenovirus was also injected into the LC of male Sprague-Dawley rats. GFP was highly expressed in the injected side of the LC and in adjacent regions. Some GFP-positive fibers were distributed in the lower lateral side of the midbrain. Immunoflourescent staining for WGA showed several WGA-positive (red) neurons that colocalized with GFP fluorescence. Immunohistochemical staining for WGA using DAB solution showed the presence of WGA-immunoreactive neurons in the injected side of the LC. The contralateral side of the LC showed no WGA-immunoreactive neurons (Fig. 4). In order to confirm the colocalization of WGA with GFP, we captured Z-stack images 3 days after TH-WGA-CMV-GFP recombinant adenovirus injection into the VTA. GFP was highly expressed in the injected side of the VTA. Many GFP-positive fibers were distributed among the GFP-positive cell bodies. WGA immunohistochemistry revealed the presence of several red fluorescent WGA-positive neurons. WGA-positive fibers and cell bodies were distributed in adjacent regions. Z-stack images showed that WGA colocalized with GFP in the VTA (Fig. 5).
Discussion
The CNS is composed of billions of neurons interconnected to form intricate neuronal circuits (Boldogkoi et al., 2004; Livet et al., 2007). Neuronal tracing has been used to identify and characterize neuronal pathways and functions in specific neuronal circuits of the complex nervous system (Yoshihara 2002). Gene promoter systems that drive high-level, cell-type-specific tracer gene expression are of great value because they allow specific cell types to endogenously express tracers, leading to visualization of specific neural circuitry of the brain. Once the tracer gene has been delivered to the target neuron, expression level and specificity is dependent upon the activity of the promoter elements incorporated into the vector used for gene delivery.
Previously, several nonspecific promoters have been used for gene delivery. The CMV promoter has been widely used to drive expression of various transgenes. Kissa et al. used the CMV promoter in an adenoviral vector for delivery of the GFP-TTC gene for in vivo neuronal tracing (Kissa et al., 2002). The CMV immediate enhancer/β-actin (CAG) promoter was shown to promote higher levels of transgene expression in several cell lines compared with the CMV and β-actin promoters (Niwa et al., 1991). Infusion of a WGA-expressing adenovirus under control of the CAG promoter through mouse nostrils led to infection and WGA expression in various cell types throughout the olfactory epithelium (Kinoshita et al., 2002). Other promoters, such as the NSE promoter (Navarro et al., 1999), synapsin-1 promoter (Kügler et al., 2001), and the human PDGF β-chain promoter, have been combined with the CMV promoter (Liu et al., 2004) to promote neuronal expression of transgenes. These promoters are useful for robust expression of tracers in the target regions, but their utility is limited due to a lack of specificity. Recently, Livet et al. (2007) generated thy-1-brainbow mice lines, in which most mice exhibited coexpression of multiple colors in individual cells in the brain.
We previously developed a synthetic promoter driving transgene expression in catecholaminergic neurons (Oh et al., 2009). In order to trace the neuronal circuit of midbrain dopaminergic neurons, we sought to use the TH upstream promoter. Toward this goal, we investigated the use of the 2.5 kb rat TH upstream promoter as a dopaminergic neuron specific promoter.
In this study, we observed cell-type-specific expression of a transgene in vitro and in vivo. In cultured HEK293 cells, GFP was highly expressed under control of the CMV promoter. However, RFP, under control of the cell-type-specific TH promoter, was not expressed in HEK293 cells. The CMV promoter can drive expression of various transgenes in most cells; therefore, GFP was strongly expressed in HEK293 cells, a human embryonic kidney cell line. In contrast, the TH promoter, a neuron-specific promoter, did not promote RFP expression in the kidney cell line. Injection of the TH-RFP-CMV-GFP recombinant adenovirus into the cerebellum of male Sprague-Dawley rats resulted in strong expression of GFP. However, RFP, under the control of TH promoter, was not expressed. These results suggest that the TH promoter drives highly specific expression of the transgene. Catecholaminergic neurons are not found in the cerebellum; therefore, RFP was not expressed in the cerebellar cortex.
In contrast to the cerebellum, we observed strong expression of RFP in the LC of adenovirus-injected mice. The LC is the primary source of noradrenergic output in the brain. Noradrenergic neurons express the TH enzyme for synthesis of noradrenaline. Therefore, RFP under the control of TH promoter was highly expressed in the LC. We also observed expression of WGA in the VTA and LC after injection of a recombinant adenovirus that expressed this transgene under control of the TH promoter. These regions have many dopaminergic and noradrenergic neurons, leading to high expression of WGA under control of the TH promoter. Recently, Oh et al. (2009) used the 2.5-kb rat TH promoter in adenovirus or adeno-associated virus (AAV) to express tracers specifically in dopaminergic neurons. The TH promoter has previously been shown to drive cell-type-specific gene expression in midbrain dopaminergic neurons using transgenic founder analysis (Liu et al., 1997). This vector drove prominent transgene expression in dopaminergic neurons of the SN. Therefore, our findings are consistent with these studies.
The genetic approach using the WGA transgene expressed under the control of cell-type-specific promoter elements resulted in labeling of neuronal circuits originating from a specific type of neuron. Previous studies in transgenic mice have used the TTC transgene as a retrograde tracer (Maskos et al., 2002). Although the transgenic mouse approach provides an efficient system to study gene function under a variety of physiological and pathological circumstances, this technology is rather technically challenging (Hickman-Davis & Davis, 2006). An alternative and complementary approach is direct gene transfer using stereotaxic injection of recombinant viral vectors. This approach permits convenient spatio-temporal control of transgene expression (Pilpel et al., 2009). Indeed, several recent studies have used viral-mediated gene delivery for neuronal tracing, demonstrating the effectiveness of this technique. Kinoshita et al. visualized olfactory pathways using a WGA-expressing adenoviral vector system (Kinoshita et al., 2002). They generated a recombinant adenovirus expressing WGA under the control of the CAG promoter. Recent studies have used cell-type-specific promoters to direct expression of GFP-WGA to midbrain dopaminergic neurons and central noradrenergic neurons using AAV and adenovirus, respectively (Oh et al., 2009). In addition, Wickersham et al. used a genetically modified rabies virus for monosynaptic restriction of viral transsynaptic tracing (Wickersham et al., 2007a; Wickersham et al., 2007b).
In this study, we used the TH promoter for cell-type-specific expression of a tracer transgene and the CMV promoter for non-specific expression of GFP. Under the control of the CMV promoter, robust expression of the downstream transgene is promoted in most mammalian cells, including neurons. Therefore, using this virus, we can differentiate between tracer expression in adenovirus-infected, GFP-expressing cells and that in non-infected cells through the combined use of the CMV and TH promoters. WGA was has been widely used as a transsynaptic tracer. Therefore, adenoviral vectors containing the WGA cDNA downstream of neuron-specific promoter elements will likely prove useful for restricted expression of WGA in a region- and cell-type-specific manner.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download