Abstract
Nitric oxide is one of many proinflammatory mediators that are involved in temporomandibular joint (TMJ) inflammatory disorder and is synthesized by inducible nitric oxide synthase (iNOS). iNOS is transcriptionally regulated by nuclear factor-κB (NF-κB) in cases of inflammation, proliferation, and apoptosis. It has also been reported that nitric oxide is positively regulated by carrageenan and negatively regulated by hyaluronan in the knee joint. The aim of this study was to histologically evaluate how inflammation and cell proliferation of the synovial membrane are affected by the exogenous administration of carrageenan and hyaluronan in the rat TMJ by investigating iNOS, NF-κB, and anti proliferating cell nuclear antigen (PCNA) immunoreactivity. As results, immunoreactive cells to iNOS, NF-κB, and PCNA were normally localized only in the synovial membrane of wild type TMJs. The numbers of immunoreactive cells were extensively larger in the carrageenan-injected synovial membranes exhibiting excessive folding, and smaller in the hyaluronan-injected synovial membranes showing a few folds. These results indicate that a carrageenan injection induced inflammation and cell proliferation especially in the synovial membrane and that hyaluronan relieved the inflammation by decreasing inflammatory molecules in the synovial membrane.
The temporomandibular joint (TMJ) is a complex structure found only in mammals and is essential for jaw movement. Its major components include the temporal bone, the condylar head of the mandible, and a fibrocartilagenous disc located between these bones that divide the joint cavity into upper and lower compartments.
TMJ inflammatory disorder causes continuous pain, which is serious in some cases, and it represents a temporomandibular disorder that can produce central-nerve-simulating effects such as periodic muscle spasm, hyperalgesia, and referred pain. TMJ disorder involves the soft tissues, including the synovial membrane, capsule, and retrodiscal tissue and the hard tissues, including the mandibular condyle and its covering cartilage, and causes transformation of the extracellular matrix.
Many proinflammatory mediators are involved in joint inflammatory disorders, such as nitric oxide, cytokines, eicosanoids, nitric oxide, biogenic amines, neuropeptides, chemokines, interferon gamma, NGFs (nerve growth factors), proteases, the coagulation pathway, the kinin system, the complement pathway, and the fibrinitric oxidelytic reaction. Among these, nitric oxide is synthesized from L-arginine by inducible nitric oxide synthase (iNOS) and is an important factor in immunological and inflammatory processes (Moncada 1992). The activation of iNOS is evidently important in inflammatory arthritis (Farrell 1992; Pelletier et al., 1999). iNOS has been detected in the synovial tissue of a TMJ with internal derangement (Takahashi et al., 1996; Homma 2001). Furthermore, the volume of iNOS increases in the synovial membrane of rats after being exposed to mechanical stimulation, and iNOS is observed in the TMJ synovial membrane in cases of internal derangement and bone arthritis (Takahashi et al., 2003; Yamaza & Masuda, 2003).
Nuclear factor-κB (NF-κB), a protein complex controlling the transcription of the κ-light-chain gene in B lymphocytes, is also related with the inflammation and immunity of arthritis (Collantes et al., 1998; Yamasaki et al., 2001; Feldmann et al., 2002). NF-κB in fibroblast-like synovial cells transcriptionally regulates iNOS in cases of inflammation, proliferation, and apoptosis (Makarov 2001; Singh et al., 2002).
Carrageenan is a large molecule and is widely used to artificially induce inflammation in the joints of animal models. Many scientists have used carrageenan to induce artificial arthritis (Lunderberg et al., 1996; Tonussi & Ferreira, 1999; Hansra et al., 2000; Egan et al., 2002; Radhakrishnan et al., 2003).
Hyaluronan, a synovial fluid constituent, has recently been applied as a treatment for joint disorders. Hyaluronan protects cartilage from damage by increasing the production of proteoglycan aggregate reduces pain by increasing PGE2 and bradykinin levels in the synovial fluid. Hyaluronan has been actively used as a treatment for TMJ patients (Kopp et al., 1991).
It has been suggested that nitric oxide is positively regulated by carrageenan and negatively regulated by hyaluronan in the knee joint (Lawand et al., 2000; Díaz-Gallego et al., 2005). However, the relationship between carrageenan and hyaluronan in TMJ disorder has not been reported yet. Furthermore, the relationship of NF-κB with carrageenan and hyaluronan has also not been reported in TMJ disorder. The aim of this study was to elucidate and visualize the effects of carrageenan and hyaluronan on inflammation and cell proliferation in TMJ disorder. High molecular weight exogenous hyaluronan was injected into rat TMJs, in which synovitis had already been induced artificially by carrageenan injection, and the morphological changes in the synovial membrane and the localization of iNOS and NF-κB in the synovial membrane was observed using immunohistochemistry. The effect of hyaluronan on cell proliferation was also studied.
All experiments were performed according to the guidelines of the Yonsei University, College of Dentistry, Intramural Animal Use and Care Committee.
Adult rats were housed in a temperature-controlled room (25℃) under artificial illumination (lights on from 05 : 00 to 17 : 00) and at 55% relative humidity with ad libitum access to food and water. Twenty six-week-old outbred rats (Samtako Bio Korea, Seoul, Korea) were divided into the following four groups, each containing five rats; Group 1: wild type, no injection; Group 2: Saline injection; Group 3: Carrageenan injection; Group 4: Carrageenan injection followed by a hyaluronan injection 1 week later.
Ketamine (30 mg; Yuhan, Korea), which is a fast-working general anesthetic for animals, was injected into the abdominal cavity. Where applicable, drugs were injected using an insulin syringe at a point 2 mm from the outer ear and 1 mm above the line that connects the outer ear and orbit. Solutions were injected into the upper joint cavity of the TMJ. Rats in all groups were sacrificed by cervical dislocation at 2 weeks after the first injection. We used 3% carrageenan and hyaluronan in this study. Carrageenan (0.01 ml of 3%; Sigma-Aldrich, St. Louis, MO, USA) was injected into both sides of the TMJ with a 32 G needle to artificially induce inflammation in the joints. Hyaluronan (LG Chemistry, Seoul, Korea) was injected at 0.04 ml/kg in the same manner; it is mainly comprised of sodium hyaluronate (10 mg/ml), and the minor components are sodium monohydrate phosphoric acid and sodium bihydrate phosphoric acid.
This experiment employed perfusion fixation. Rats were anesthetized by ether inhalation, and 4% paraformaldehyde was used as the fixation solution. The abdominal region was opened with scissors, the chest was exposed, and the left ventricle was poked with a needle. The right auricle of heart was then cut with scissors to allow blood to escape. Phosphate-buffered saline (0.05 M, PBS) was administered using a peristaltic pump at 30~40 ml per minute. When clear water exited from the right auricle, the PBS was replaced with fixation solution, which was administered for 5~10 minutes. After fixation, the head was removed from the body and was fixed for 4~8 hours using the same fixation solution as that used for peristaltic pumping. The specimen was treated with 10% EDTA (Duksan, Korea) for 4 weeks, followed by washing with water, and drying. The tissue was made transparent and cast into a gelatin block using 7.5% gelatin in 15% sucrose for staining.
Samples were sectioned at a thickness of 7 µm for immunohistochemical staining. Slides were placed in PBS for 30 minutes to remove the gelatin. After being washed twice with distilled water for 5 minutes, the sections were blocked in 0.3% hydrogen peroxide for 15 minutes. The slides were incubated with rabbit polyclonal antibodies against NF-κB (1 : 400, Santa Cruz Biotechnology, Santa Cruz, CA, USA), iNOS (1 : 100, Santa Cruz Biotechnology), and mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) (1 : 200, Neomarkers Inc., Freemont, CA, USA), as primary antibodies at 4℃ overnight. After washing with PBS, the specimens were reacted with biotinylated goat antirabbit secondary antibody, mouse secondary antibody (Zymed, San Francisco, CA, USA) and streptavidin peroxidase (Zymed) at room temperature for 10 minutes each. Finally, the specimens were visualized using a diaminobenzidine reagent kit (Zymed). The immunostained sections were counterstained with hematoxylin.
Several folds, which are process-like structures, exist in the posterior-superior synovial membrane of the upper joint cavity. The length and number of folds increase with inflammation in TMJ disorder (Nozawa-Inoue et al., 2003; Tzaribachev et al., 2009). Coincidently, a large fold was observed in the posterior portion of the synovial membrane of the carrageenan-injected TMJs (Fig. 1C), whereas a small fold was observed in the wild type and saline-injected TMJs (Figs. 1A and B). TMJs serially injected with carrageenan and hyaluronan (carrageenan/hyaluronan-injected TMJ) exhibited a few folds, which were larger and smaller in size than that of the wild type TMJs and the carrageenan-injected TMJs, respectively (Fig. 1D). These results indicate that carrageenan induced the folding of the synovial membrane in the TMJs, and that the hyaluronan reduced the folding of the synovial membrane that had been induced by carrageenan.
To clarify the effect of carrageenan and hyaluronan on the synovial membrane of TMJ, we examined changes in the localizing patterns of iNOS and NF-κB in the synovial membrane after injecting carrageenan and hyaluronan. It has been reported that iNOS is localized in the synovial membrane of normal rat TMJs, as nitric oxide also acts as a mediator to maintain the physiological condition of the TMJ (Yamaza et al., 2004). In our study, iNOS immunoreactivity was detected in the synovial membrane of the posterior-superior portion of the articular cavity in the wild type and saline-injected TMJs (Figs. 1A and B). The immunoreactive cells to iNOS were extensively observed through the enlarged synovial folds and synovial membranes of the carrageenan-injected TMJs compared to the other group of TMJs (Fig. 1C). The synovial membranes in the carrageenan/hyaluronan-injected TMJs also exhibited iNOS immunoreactivity, but the numbers of immunoreactive cells were much smaller than in the carrageenan-injected TMJs (Fig. 1D). This result confirms that iNOS immunoreactive cells in synovial membranes increased by carrageenan treatment and decreased following hyaluronan treatment.
NF-κB immunoreactivity in each group shared the iNOS localization patterns of its group (Fig. 2A and B). NF-κB immunoreactive cells were observed extensively in the synovial membranes of the carrageenan-injected TMJs but not extensively in the carrageenan/hyaluronan-injected TMJs (Figs. 2C and D). This result suggests that NF-κB immunoreactive cells in the synovial membrane increased following carrageenan treatment and decreased following hyaluronan treatment.
The result that iNOS and NF-κB immunoreactive cells were extensively observed in the largest fold of the carrageenan-injected TMJs leads us to define the relationship between inflammation and cell proliferation using PCNA immunostaining. PCNA immunoreactive cells were observed in carrageenan-injected TMJs rather than in any other TMJ groups (Fig. 3).
The intensity of folding and the number of immunoreactive cells to iNOS, NF-κB, and PCNA in the synovial membranes is summarized in Table 1 and by utilizing the semi-quantitative scoring method (Kishimoto et al., 2007; Min et al., 2007).
The synovial membrane covers the anterior and posterior portion of the upper and lower joint cavities except the articular disc and articular surface. The length and number of folds in the posterior synovial membrane increase with the inflammation of TMJ (Nozawa-Inoue et al., 2003; Tzaribachev et al., 2009). In our study, while small folds were observed in the wild type and saline-injected TMJs, large folds were observed in the synovial membrane of the carrageenan-injected TMJs, suggesting a close relationship of carrageenan with synovial folding and inflammation in the TMJ synovial membrane. Previously, it has been suggested that carrageenan induces inflammation in paw edema and lung pleurisy by increasing NF-κB and iNOS levels (Cuzzocrea et al., 1999; Cuzzocrea et al., 2000; Al-Majed et al., 2003). In our study, iNOS and NF-κB immunoreactive cells increased extensively in the membranes of the carrageenan-injected TMJs, which confirms that carrageenan induces inflammation in the TMJ synovial membrane by positively regulating both iNOS and NF-κB. However, after the hyaluronan injection the number of iNOS and NF-κB immunopositive cells decreased, and the size of the synovial folding was reduced. These results indicate that hyaluronan relieved the inflammation in the synovial membrane by negatively regulating iNOS and NF-κB. Additionally, the co-localizing pattern of NF-κB and iNOS in the synovial membrane of each group supports a previous suggestion that NF-κB transcriptionally regulates iNOS in cases of inflammation, proliferation, and apoptosis (Makarov 2001; Singh et al., 2002).
The number of synovial membrane cells increased in carrageenan-injected TMJs, which lead us to define the relationship between inflammation and cell proliferation. The finding that proliferating cells displaying PCNA immunoreactivity were extensively observed in the enlarged synovial membrane of the carrageenan-injected TMJs suggests that carrageenan is associated with cell proliferation and inflammation and that cell proliferation induces synovial membrane folding. This is coincident with previous suggestions that carrageenan induces not only inflammation in the joints but also cell proliferation in the colon (Calvert & Reicks, 1988; Wilcox et al., 1992; Suzuki et al., 2000).
In summary, TMJ synovial cells were normally immunoreactive to iNOS, NF-κB, and PCNA, and carrageenan elevated iNOS, NF-κB, and cell proliferation resulting in an increase in synovial cell number and inflammation, which was relieved by hyaluronan. This study is the first report demonstrating immunohistochemical evidence confirming the opposing roles of hyaluronan and carrageenan by modulating molecules related to inflammation and cell proliferation in TMJ.
Figures and Tables
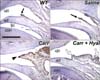 | Fig. 1Inducible nitric oxide synthase (iNOS) immunoreactivity at the synovial membrane. The upper joint cavity (ujc) and lower joint cavity (ljc) are separated by the articular disc (ad). (A and B) Wild type temporomandibular joint (TMJ) shows brown-colored immunoreactive cells at the synovial membrane in the posterior portion of the upper joint cavity. The saline-injected TMJ (Saline) shares the iNOS localization pattern with the wild type TMJ. A small fold is observed in the synovial membrane of the wild type and saline-injected TMJs (black arrow). (C) A large fold (black arrowhead), including many iNOS immunopositive cells is observed in the carrageenan-injected TMJ (Carr). (D) A few small folds (white arrows) are found in the TMJ injected with carrageenan followed by hyaluronan (Carr+Hyal). The number of immunoreactive cells in the fold is less than that of the carrageenan-injected TMJs. Scale bar: 500 µm. con: condyle. |
 | Fig. 2Nuclear factor-κB (NF-κB) immunoreactivity at the synovial membrane. (A and B) Wild type and saline-injected TMJs show a small fold (black arrow) and brown-colored immunoreactive cells at the synovial membrane in the posterior portion of the upper joint cavity. The NF-κB localizing pattern in wild type TMJs is almost the same as that in saline-injected TMJs. (C) A large fold (black arrowhead), including many NF-κB immunopositive cells, can be seen in the carrageenan-injected TMJ (Carr). (D) Three small folds (white arrows) in a TMJ synovial membrane injected with carrageenan and hyaluronan (Carr+Hyal). The number of immunoreactive cells in the fold is less than that of the carrageenan-injected TMJ. Scale bar: 500 µm. |
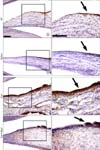 | Fig. 3Proliferating cell nuclear antigen (PCNA) immunoreactivity at the synovial membrane of the upper joint cavity. (A~H) PCNA immunopositive cells (black arrows) were detected in the posterior-superior synovial membrane. (C, G) PCNA immunoreactive cells were localized in a large area of the synovial membrane in the carrageenan-injected TMJs (Carr). (D, H) The number of PCNA immunoreactive cells in the TMJ synovial membranes injected with carrageenan and hyaluronan (Carr+Hyal) is less than that of carrageenan-injected TMJs. Scale Bars: 100 µm. |
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-331-E00318).
References
1. Al-Majed AA, Khattab M, Raza M, Al-Shabanah OA, Mostafa AM. Potentiation of diclofenac-induced anti-inflammatory response by aminoguanidine in carrageenan-induced acute inflammation in rats: the role of nitric oxide. Inflamm Res. 2003. 52:378–382.
2. Calvert RJ, Reicks M. Alterations in colonic thymidine kinase enzyme activity induced by consumption of various dietary fibres. Proc Soc Exp Biol Med. 1988. 189:45–51.
3. Collantes E, Valle Blázquez M, Mazorra V, Macho A, Aranda E, Muñoz E. Nuclear factor-kappa B activity in T cells from patients with rheumatic diseases: a preliminary report. Ann Rheum Dis. 1998. 57:738–741.
4. Cuzzocrea S, Mazzon E, Calabro G, et al. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000. 162:1859–1866.
5. Cuzzocrea S, Tan DX, Costantino G, Mazzon E, Caputi AP, Reiter RJ. The protective role of endogenous melatonin in carrageenan-induced pleurisy in the rat. FASEB J. 1999. 13:1930–1938.
6. Díaz-Gallego L, Prieto JG, Coronel P, Gamazo LE, Gimeno M, Alvarez AI. Apoptosis and nitric oxide in an experimental model of osteoarthritis in rabbit after hyaluronic acid treatment. J Orthop Res. 2005. 23:1370–1376.
7. Egan CG, Lockhart JC, Ferrell WR, Day SM, McLean JS. Pathophysiological basis of acute inflammatory hyperaemia in the rat knee: roles of cyclo-oxygenase-1 and -2. J Physiol. 2002. 539(Pt 2):579–587.
8. Farrell AJ. Increased concentration of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic disease. Ann Rheum Dis. 1992. 51:1219–1222.
9. Feldmann M, Andreakos E, Smith C, et al. Is NF-kappaB a useful therapeutic target in rheumatoid arthritis? Ann Rheum Dis. 2002. 61:Suppl 2. ii13–ii18.
10. Hansra P, Moran EL, Fornasier VL, Bogoch ER. Carrageenan-induced arthritis in the rat. Inflammation. 2000. 24:141–155.
11. Homma H, Takahasi T, Seki H, Ohtani N, Kondon T, Fukuda M. Immunohistochemical localization of inducible nitric oxide synthase in synovial tissue of human temporomandibular joints with internal derangement. Arch Oral Biol. 2001. 46:93–97.
12. Kishimoto G, Hosomichi J, Muramoto T, Kanno Z, Soma K. Influence of estrogen cycle on temporomandibular joint synovial membrane in rat with deviated mandible. J Med Dent Sci. 2007. 54:79–85.
13. Kopp S, Akerman S, Nilner M. Short-term effects of intra-articular sodium hyaluronate, glucocorticoid and saline injections on rheumatoid arthritis of the temporomandibular joint. J Craniomandib Disord. 1991. 5:231–238.
14. Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. 2000. 86:69–74.
15. Lundeberg T, Alstergren P, Appelgren A, Appelgren B, Carleson J, Kopp S. A model for experimentally induced temporomadubular joint arthritis in rats: effects of carrageenan on europeptide like immunoreactivity. Neuropeptides. 1996. 30:37–44.
16. Makarov SS. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001. 3:200–206.
17. Min HJ, Lee MJ, Kim JY, et al. Alteration of BMP-4 and Runx2 expression patterns in mouse temporomandibular joint after ovariectomy. Oral Diseases. 2007. 13:220–227.
18. Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992. 145:201–227.
19. Nozawa-Inoue K, Amizuka N, Ikeda N, Suzuki A, Kawano Y, Maeda T. Synovial membrane in the temporomandibular joint--its morphology, function and development. Arch Histol Cytol. 2003. 66:289–306.
20. Pelletier JP, Lascau-Coman V, Jovanovic D, et al. Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J Rheumatol. 1999. 26:2002–2014.
21. Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003. 104:567–577.
22. Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002. 46:2079–2086.
23. Suzuki J, Na HK, Upham BL, Chang CC, Trosko JE. Lamda-carrageenan-induced inhibition of gap-junctional intercellular communication in rat liver epithelial cells. Nutrition and Cancer. 2000. 36:122–128.
24. Takahashi T, Homma H, Nagai H, et al. Specific expression of inducible nitric oxide synthase in the synovium of the diseased temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 95:174–181.
25. Takahashi T, Kondoh T, Kamei K, et al. Elevated levels of nitric oxide in synovial fluid from patients with temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996. 82:505–509.
26. Tonussi CR, Ferreira SH. Tumor necrosis factor-alpha mediates carrageenan-induced knee joint incapacitation and also trigger overt ociception in previously inflamed rat knee joints. Pain. 1999. 82:81–87.
27. Tzaribachev N, Fritz J, Horger M. Spectrum of magnetic resonance imaging appearances of juvenile Temporomandibular Joints (TMJ) in non-rheumatic children. Acta Radiologica. 2009. 50:1182–1186.
28. Wilcox DK, Higgins J, Bertram TA. Colonic epithelial cell proliferation in a rat model of nongenotoxin-induced colonic neoplasia. Laboratory Investigation. 1992. 67:405–411.
29. Yamasaki S, Kawakami A, Nakashima T, et al. Importance of NF-kappaB in rheumatoid synovial tissues: in situ NF-kappaB expression and in vitro study using cultured synovial cells. Ann Rheum Dis. 2001. 60:678–684.
30. Yamaza T, Masuda K, Atsuta I, Nishijima K, Kido MA, Tanaka T. Oxidative stress induced DNA damage in the synovial cells of the temporomandibular joint in the rat. J Dent Res. 2004. 83:619–624.
31. Yamaza T, Masuda KF, Tsukiyama Y, et al. NF-kB activation and iNOS expression in the synovial membrane of rat temporomandibular joints after induced synovitis. J Dent Res. 2003. 82:183–188.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download