Abstract
Objective
The objective of this study was to determine the detection rate of respiratory viruses and investigate the associated factors with respiratory virus detection in newborn infants with suspected infection.
Methods
From January 2013 to December 2015, respiratory virus real-time polymerase chain reaction (RT-PCR) results were obtained from 136 newborn infants aged ≤28 days who admitted to the neonatal intensive care unit (NICU) of Dong-A University Hospital with suspected infectious diseases. We performed a retrospective analysis of the detection rate of respiratory virus, classes of respiratory viruses, clinical characteristics, and social environment characteristics associated with respiratory virus detection.
Results
Of the 136 infants, 36 infants (26.5%) had the 37 following respiratory viruses: Respiratory syncytial virus (n=23), Rhinovirus (n=10), Parainfluenza virus (n=2), Influenza virus (n=1), and Corona virus (n=1). The detection of respiratory viruses was significantly associated with the old age at admission (17.3±5.8 vs. 10.6±6.8 days), the presence of respiratory symptoms: cough (72.2% vs. 7%), rhinorrhea (63.9% vs. 10%), rale (16.7% vs. 1%), a family history of respiratory illness (38.9% vs. 13%), especially siblings’ respiratory illness (33.3% vs. 8%), and a seasonal preference (October-March) (80.6% vs. 50%) (P<0.01).
REFERENCES
1). Gerdes JS. Diagnosis and management of bacterial infections in the neonate. Pediatr Clin North Am. 2004. 51:939–59.

2). de Assis Meireles L., Vieira AA., Costa CR. Evaluation of the neonatal sepsis diagnosis: use of clinical and laboratory parameters as diagnosis factors. Rev Esc Enferm USP. 2011. 45:33–9.
3). Polin RA. the Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012. 129:1006–15.

4). Simonsen KA., Anderson-Berry AL., Delair SF., Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014. 27:21–47.

5). van den Anker JN. How to optimize the evaluation and use of antibiotics in neonates. Early Hum Dev. 2014. 90(Suppl 1):S10–2.

6). Muller-Pebody B., Johnson AP., Heath PT., Gilbert RE., Henderson KL., Sharland M, et al. Empirical treatment of neonatal sepsis: are the current guidelines adequate? Arch Dis Child Fetal Neonatal Ed. 2011. 96:F4–8.

7). Jacqz-Aigrain E., Zhao W., Sharland M., van den Anker JN. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med. 2013. 18:28–34.

8). Edmond K., Zaidi A. New approaches to preventing, diagnosing, and treating neonatal sepsis. PLoS Med. 2010. 7:e1000213.

9). Tregoning JS., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010. 23:74–98.

10). Diniz EM., Vieira RA., Ceccon ME., Ishida MA., Vaz FA. Incidence of respiratory viruses in preterm infants submitted to mechanical ventilation. Rev Inst Med Trop Sao Paulo. 2005. 47:37–44.

11). Bennett NJ., Tabarani CM., Bartholoma NM., Wang D., Huang D., Riddell SW, et al. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr. 2012. 161:814–8.

12). Piippo-Savolainen E., Korppi M. Wheezy babies-wheezy adults? Review on long-term outcome until adulthood after early childhood wheezing. Acta Paediatr. 2008. 97:5–11.

13). García-García ML., Calvo C., Casas I., Bracamonte T., Rellán A., Gozalo F, et al. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr Pulmonol. 2007. 42:458–64.

14). Jackson DJ., Gangnon RE., Evans MD., Roberg KA., Anderson EL., Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008. 178:667–72.

15). Lemanske RF Jr., Jackson DJ., Gangnon RE., Evans MD., Li Z., Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005. 116:571–7.
16). Fjaerli HO., Farstad T., Rød G., Ufert GK., Gulbrandsen P., Nakstad B. Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC Pediatr. 2005. 5:31.

17). Sigurs N., Gustafsson PM., Bjarnason R., Lundberg F., Schmidt S., Sigurberg-sson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005. 171:137–41.

18). Kuypers J., Wright N., Ferrenberg J., Huang ML., Cent A., Corey L, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006. 44:2382–8.

19). Freymuth F., Vabret A., Cuvillon-Nimal D., Simon S., Dina J., Legrand L, et al. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J Med Virol. 2006. 78:1498–504.

20). Templeton KE., Scheltinga SA., Beersma MF., Kroes AC., Claas EC. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004. 42:1564–9.

21). Bellau-Pujol S., Vabret A., Legrand L., Dina J., Gouarin S., Petitjean-Lecher-bonnier J, et al. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005. 126:53–63.

22). Kim SR., Ki CS., Lee NY. Rapid detection and identification of 12 respiratory viruses using a dual priming oligonucleotide system-based multi-plex PCR assay. J Virol Methods. 2009. 156:111–6.

23). Kwak YH., Choi EH., Lee HJ. Detection of rhinovirus from children with lower respiratory tract infections by reverse transcription polymerase chain reaction. Infect Chemother. 2003. 35:1–11.
24). Lim JS., Woo SI., Kwon HI., Baek YH., Choi YK., Hahn YS. Clinical characteristics of acute lower respiratory tract infections due to 13 respiratory viruses detected by multiplex PCR in children. Korean J Pediatr. 2010. 53:373–9.

25). López-Alarcón M., Villalpando S., Fajardo A. Breast-feeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. J Nutr. 1997. 127:436–43.
26). César JA., Victora CG., Barros FC., Santos IS., Flores JA. Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: nested case-control study. BMJ. 1999. 318:1316–20.

27). Hong SJ., Kim DK., Lee DS., Cho SM., Choi SM. RSV outbreak at a single postpartum care center in Gyeongsangbukdo. Korean J Perinatol. 2016. 27:60–6.

Fig. 1
Respiratory virus detection classes. RT-PCR, real-time polymerase chain reaction; RSV, respiratory syncytial virus.
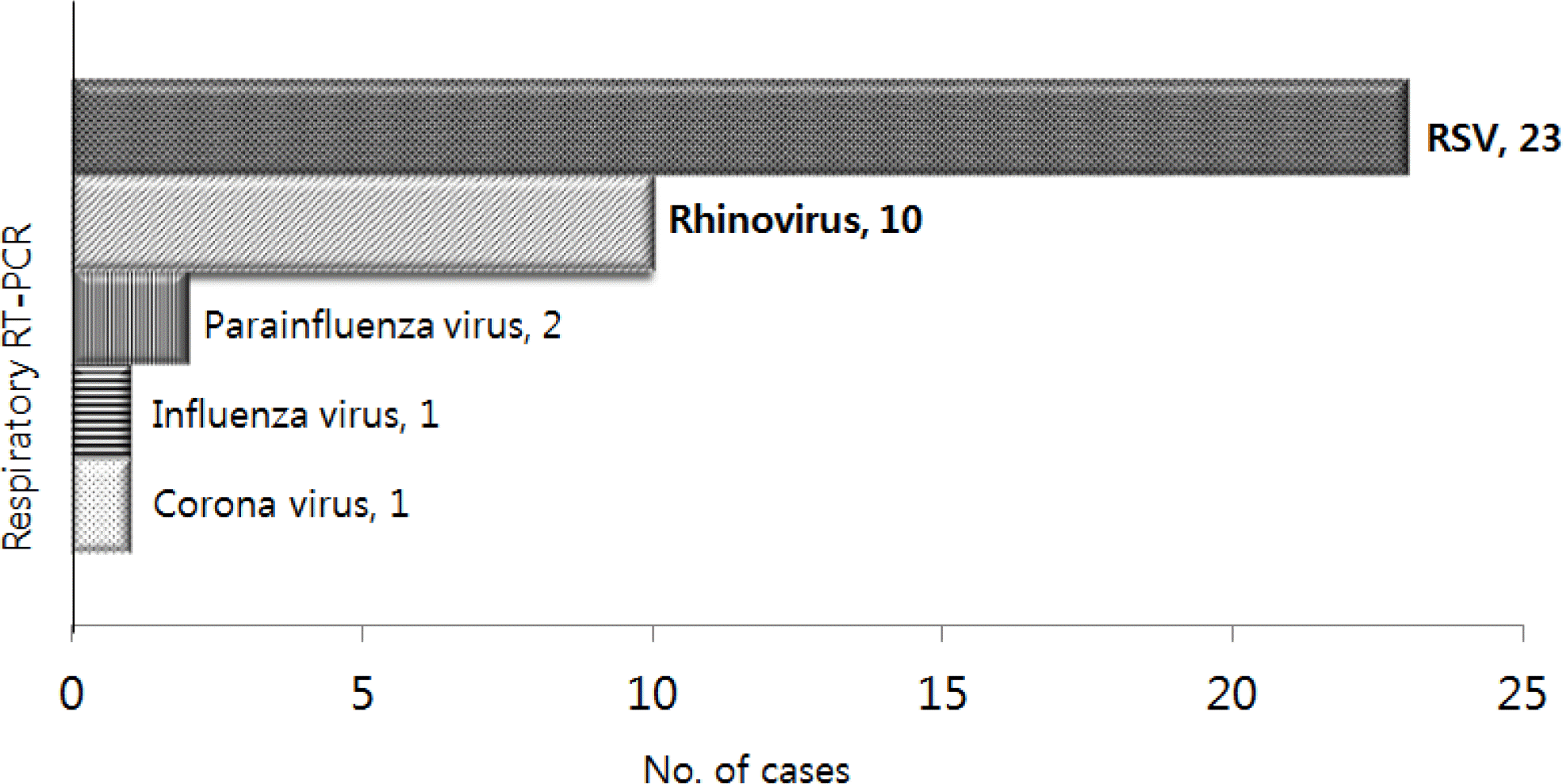
Fig. 2
Monthly distribution of respiratory virus detection cases. PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
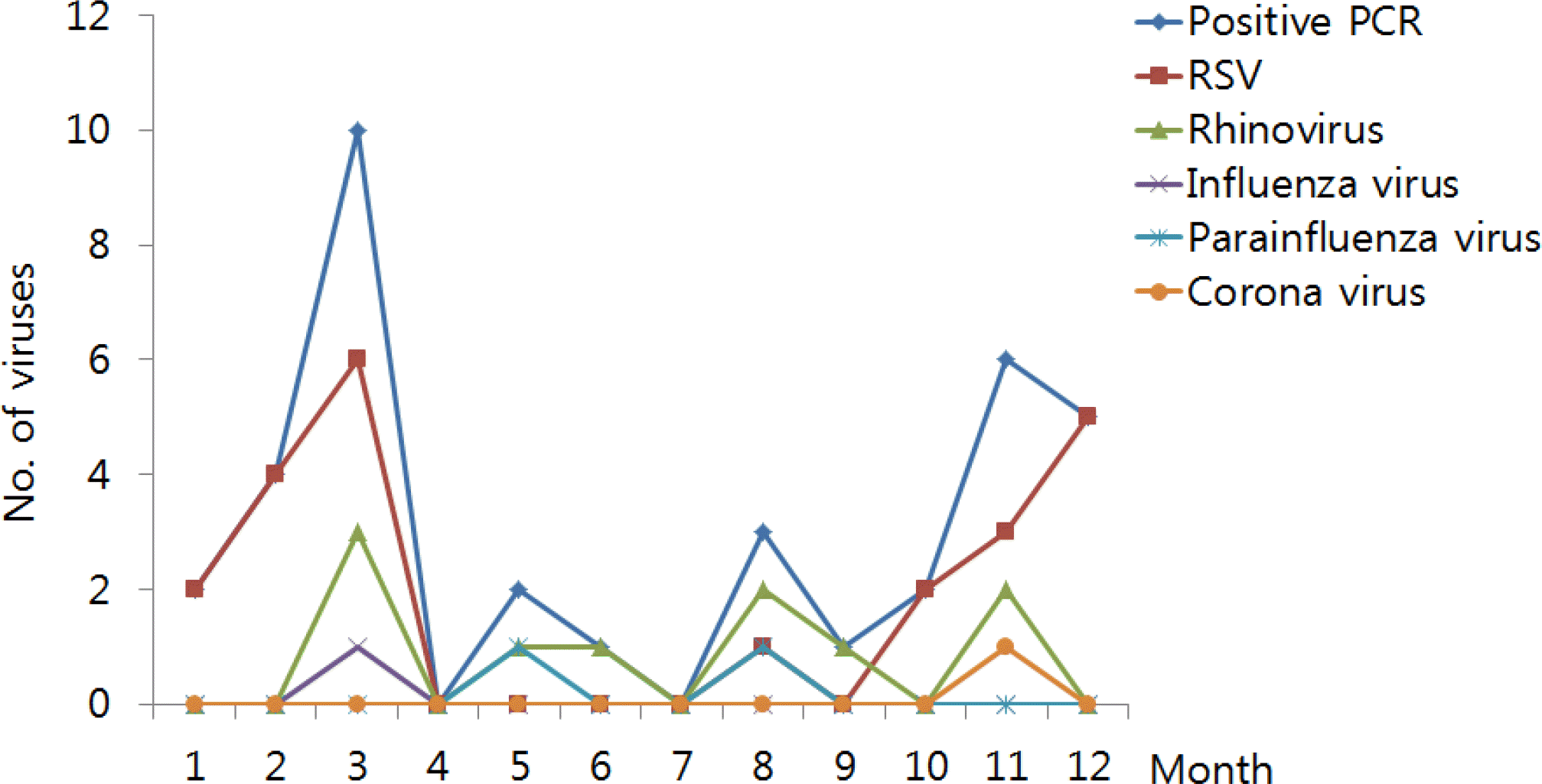
Table 1.
Clinical Characteristics Related with Respiratory Virus Detection
Table 2.
Socio-Environment Characteristics Related to Respiratory Virus Detection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download