Abstract
Objective
Methods
Results
REFERENCES
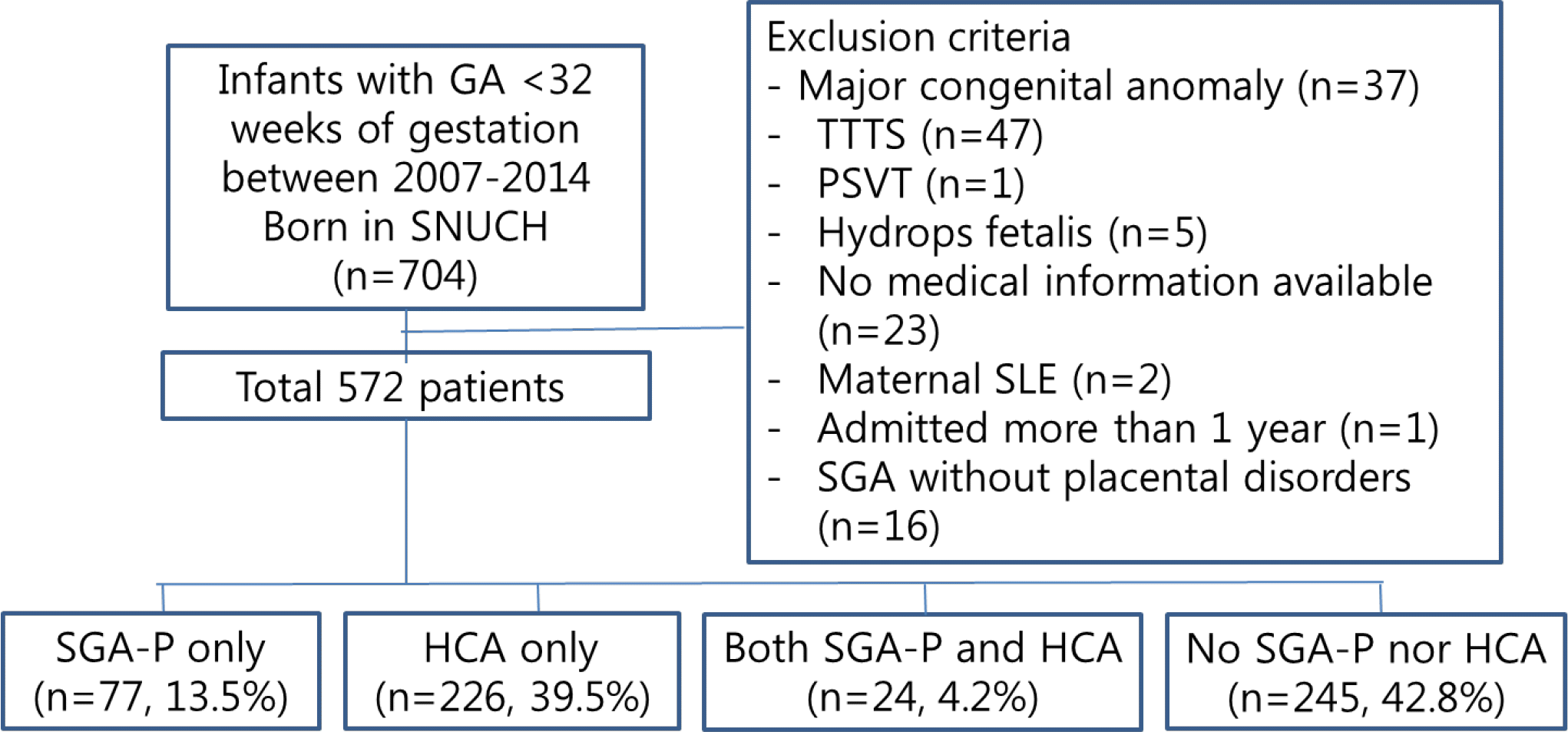 | Fig. 1Flow chart of the study population. A total of 572 patients with <32 weeks of gestation born between 2007 and 2014 were included in the analysis. 77 patients (13.5%) were in group 1, 226 patients (39.5%) were in group 2, and 24 patients (4.2%) were in group 3. GA, gestational age; SNUCH, Seoul National University Children's Hospital; TTTS, twin to twin transfusion syndrome; PSVT, paroxysmal supraventricular tachycardia; SLE, systemic lupus erythematosus; SGA-P; small for gestational age with placental disorders; HCA, histologic chorioamnionitis. |
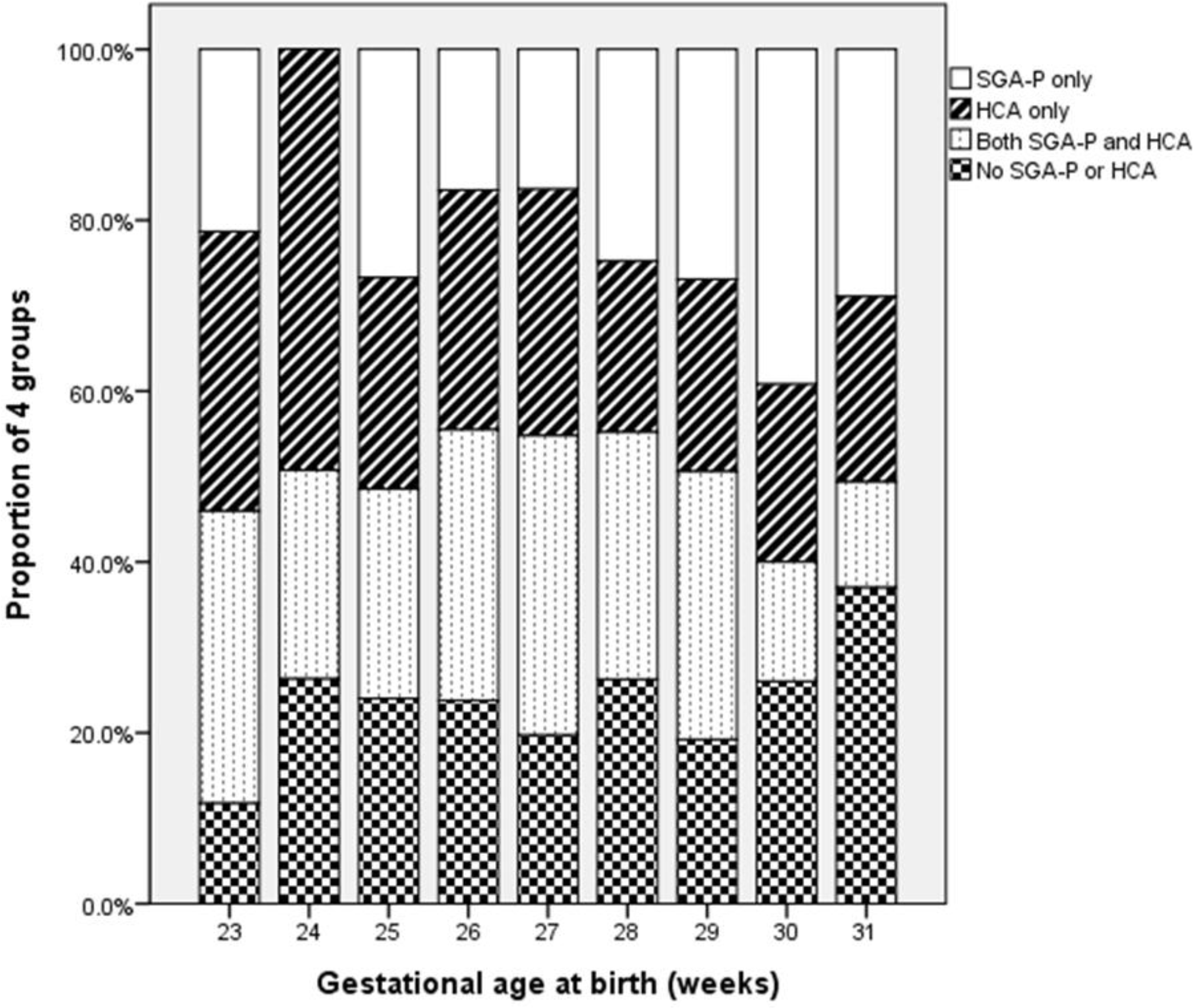 | Fig. 2The proportion of 4 groups according to the gestational age at birth. The percentage of histologic chorioamnionitis group was decreased and the percentage of small for gestational age with placental disorders was increased as the gestational age gets older (P=0.045). SGA-P, small for gestational age with placental disorders; HCA, histologic chorioamnionitis. |
Table 1.
| SGA-P only (n=77) | HCA only (n=226) | Both SGA-P and HCA (n=24) | No SGA-P nor HCA (n=245) | P-value | |
|---|---|---|---|---|---|
| Gestational age | 29+2 (27+4, 30+3) | 28+0 (26+0, 30+2)∗ | 27+6 (26+1, 29+6) | 29+0 (27+0, 30+6) | 0.002 |
| Birth weight (g) | 710 (540, 890)∗ | 1,085 (845, 1,370)∗ | 640 (538, 795)∗ | 1,220 (920, 1,490) | <0.001 |
| Male | 36 (46.8) | 111 (49.1) | 11 (45.8) | 143 (58.4) | 0.118 |
| Multiple pregnancy | 23 (29.9)∗ | 112 (49.6)∗ | 5 (20.8)∗ | 167 (68.2) | <0.001 |
| Cesarean section | 70 (90.9)∗ | 99 (43.8)∗ | 21 (87.5)∗ | 144 (58.8) | <0.001 |
| Antenatal steroid use before 12 hours after birth | 62 (80.5) | 175 (77.4) | 17 (70.8) | 188 (76.7) | 0.784 |
| Preeclampsia | 39 (50.6)∗ | 7 (3.1)∗ | 15 (62.5)∗ | 26 (10.6) | <0.001 |
| PROM>18 hours | 12 (15.6)∗ | 148 (65.5) | 6 (25.0)∗ | 141 (57.6) | <0.001 |
| GDM | 4 (5.2) | 12 (5.3) | 1 (4.2) | 11 (4.5) | 0.960 |
| Cord pH | 7.24 (7.18, 7.29)∗ | 7.32 (7.28, 7.35) | 7.23 (7.15, 7.27)∗ | 7.32 (7.27, 7.35) | <0.001 |
| 1 minute Apgar score | 3 (2, 5)∗ | 5 (2, 6) | 3 (1, 5) | 5 (2, 6) | 0.002 |
| 5 minute Apgar score | 6 (5, 7)∗ | 7 (5, 8) | 6 (3, 7)∗ | 7 (5, 8) | 0.003 |
Table 2.
| SGA-P only (n=77) | HCA only (n=226) | Both SGA-P and HCA (n=24) | No SGA-P nor HCA (n=245) | P-value | |
|---|---|---|---|---|---|
| Mortality | 14 (18.2)∗ | 19 (8.4) | 6 (25.0)∗ | 18 (7.3) | 0.004 |
| Surfactant use | 43 (55.8) | 100 (44.2) | 16 (66.7) | 111 (45.3) | 0.069 |
| PDA treatment | 41 (53.2) | 126 (55.8) | 16 (66.7) | 120 (49.0) | 0.253 |
| Pharmacological | 38 (49.4) | 110 (48.7) | 14 (58.3) | 106 (43.3) | 0.387 |
| Surgical | 7 (9.1) | 49 (21.7) | 6 (25.0) | 46 (18.8) | 0.066 |
| Moderate or severe BPD at 36 weeks | 30 (45.5)∗ | 72 (34.4) | 6 (30.0) | 58 (25.1) | 0.011 |
| Severe BPD at 36 weeks | 13 (19.7) | 27 (12.9) | 4 (20.0) | 29 (12.6) | 0.348 |
| Sepsis | 23 (29.9) | 60 (26.5) | 6 (25.0) | 44 (18.0) | 0.067 |
| Early sepsis | 6 (7.8) | 16 (7.1) | 1 (4.2) | 8 (3.3) | 0.172 |
| Late sepsis | 20 (26.0) | 51 (22.6) | 6 (25.0) | 41 (16.7) | 0.196 |
| NEC≥stage 2b | 9 (11.7) | 15 (6.6) | 1 (4.2) | 15 (6.1) | 0.392 |
| IVH≥grade 3 | 4 (5.2) | 19 (8.4) | 4 (16.7) | 16 (6.5) | 0.244 |
| ROP with surgery or VEGF | 9 (13.2) | 39 (18.5) | 4 (20.0) | 31 (13.3) | 0.394 |
| iNO use <14 days from birth | 6 (7.8) | 17 (7.5) | 1 (4.2) | 15 (6.1) | 0.885 |
| Pulmonary hypertension | 11 (14.3) | 14 (6.2) | 4 (16.7) | 17 (6.9) | 0.046 |
| Duration of hospital stay | 60 (43, 94) | 64 (39, 88) | 65 (41, 106) | 54 (35, 79) | 0.209 |
| Discharge with respiratory support | 18 (23.4) | 59 (26.1) | 6 (25.0) | 43 (17.6) | 0.154 |
Values are presented as number (%) or median (interquartile range). Abbreviations: SGA-P, small for gestational age with placental disorders; HCA, histologic chorioamnionitis; PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; VEGF, vasculo-endothelial growth factor; iNO, inhaled nitric oxide.
Table 3.
Adjusted for Gestational age at birth (weeks), cesarean section, 5 minute Apgar score, multiple pregnancy, premature rupture of membranes before 18 hours prior to delivery, and preeclampsia. Abbreviations: SGA-P, small for gestational age with placental disorders; HCA, histologic chorioamnionitis; aOR, adjusted odds ratio; CI, confidence interval; BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; VEGF, vasculo-endothelial growth factor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download