Abstract
Brain death is defined as the complete and irreversible loss of cerebral and brainstem function while the heart continues to beat. Unlike cardiac death, brain death is associated with medical, legal, and ethical issues. Common causes of neonatal brain death are perinatal asphyxia, birth trauma, cerebral infection, malformations, severe intracranial hemorrhage, and metabolic diseases. Although a diagnosis of brain death is usually based on clinical criteria, it is not well defined in neonates, especially in preterm babies. Furthermore, there are no guidelines on life expectancy, prolonging life care, or discontinuation of life support in brain dead newborns in Korea. We report a case of brain death due to hypoxic ischemic encephalopathy in a near-term newborn, present the need to establish guidelines for brain death in newborns based on national standards, and discuss further directions about end of life treatment after brain death.
Brain death is defined as the irreversible and final cessation of cerebral and brainstem function. According to a previous study of patients in the pediatric intensive care unit, neonatal brain death accounted for 2.14% of total death.1 Studies of brain death exclusively in newborns have not been reported. Although determination of brain death in newborns is based mainly on clinical examination, there are no specific national or international criteria due to lack of knowledge, especially in premature infants whose gestational age is <37 weeks. In addition, references for life expectancy and guidelines on prolonging life care or withholding treatment after brain death do not yet exist in Korea.
Herein, we report a case of brain death due to hypoxic ischemic encephalopathy in a near-term newborn who expired on the 27th day of age, and investigate current issues related to neonatal brain death.
A male was born prematurely at 36+2 weeks of gestational age, weighing 2,620 g. He was the second baby of dichorionic diamniotic twins. After vaginal delivery of the first baby, he was delivered via emergency Cesarean section due to fetal distress. Apgar score was 1, 1, and 2 at 1, 5, and 10 minutes, respectively. At birth he showed no spontaneous respiratory effort, no movement, generalized cyanosis, and bradycardia, so he was resuscitated with intubation, cardiac massage, and intratracheal epinephrine. After 15 minutes of resuscitation, only the heart rate was increased to more than 100 bpm.
The mother was a 41-year-old nullipara, impregnated via in vitro fertilization. She was admitted at 30 weeks gestation with preterm labor and administered two doses of betamethasone. She had no past medical, smoking, or alcoholic history.
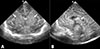
On neonatal intensive care unit (NICU) admission, the baby showed a heart rate of 156 bpm, blood pressure of 58/40 mmHg, and body temperature of 36.3℃ without spontaneous respiration. Body profile measurements included birth weight 2,620 g (50-75th percentile), height 48 cm (50-75th percentile), and head circumference 35.1 cm (>90th percentile). He showed no spontaneous breath, no movement, and no response to stimulation. Both pupils were fixed and dilated at 4.5 mm without light and corneal reflexes. Slight edema was observed on the whole body, but there was no evidence of fetal hydrops. Initial venous blood gas analysis was pH 6,752, pCO2 94.6 mmHg, HCO3- 13.3 mmol/L, and base excess -22.3 mmol/L. The baby, assisted with mechanical ventilator, was treated for respiratory and metabolic acidosis, hypotension, oliguria, and electrolyte imbalances such as hyperkalemia, hyponatremia, and hypocalcemia. Phenobarbital was started for bizarre, generalized clonic seizures that appeared at 3 hours after birth, and discontinued the next day due to absence of any movement including respiratory effort. Brain ultrasonography showed severe hypoxic ischemic encephalopathy (Fig. 1) and magnetic resonance imaging was not done because he was completely dependent on mechanical respiratory support. Echocardiogram showed normal cardiac function with good contractility. Bowel sound was not auscultated with gasless abdomen on abdominal radiograph. Abdominal ultrasonography demonstrated no remarkable findings except for liquefaction of adrenal hematoma. Results of tests for metabolic disorders were all negative. We started empirical antibiotic treatment on the first day with ampicillin and cefotaxime, but stopped the medications on the 4th day of life after confirmation of no bacterial growth in the blood culture. We fed the baby via a nasogastric tube from the 9th day of life and stopped feeding on the 15th day of life due to aspiration pneumonia.
Clinically, the patient was comatose, apneic, and atonic without any change, and consistently showed absence of brainstem reflexes (light reflex of pupils, gag or sucking reflexes, corneal reflex, oculovestibular reflex, and auditory brainstem response). The clinical features were staged III by modified Sarnat and Sarnat stages of hypoxic ischemic encephalopathy.2 We excluded any reversible conditions that could interfere with neurologic examination, such as hypothermia, hypotension, metabolic disturbances, and use of medications. On the 7th day of life, an apnea test was attempted with blood gas analysis of pH 7.436, pCO2 35.6 mmHg before changing to continuous positive airway pressure, but it had to be terminated due to bradycardia and oxygen desaturation without any respiratory effort. Although ancillary tests such as bedside electroencephalogram or radionuclide cerebral blood flow were not available in our unit, the patient's neurologic examinations were consistent with the condition of brain death, based on the evaluation of two neonatologists at intervals of more than 24 hours. The parents had consistently demanded discontinuation of all treatments from the first week of life, and medical staff had been continuously counseling the parents regarding the balance between the infant's best interest and treatment burden, the end-of-life decision-making process, and end-of-life treatment. The ethics committee was summoned and it was decided to withdraw treatment. On the 27th day, the mechanical ventilator was removed and the patient died of cardiac arrest in 10 minutes after cessation of ventilator care.
Brain death is a biological phenomenon that causes complete and irreversible loss of cerebral and brainstem function. It is based on three neurologic findings: coma or unresponsiveness, absence of brainstem reflexes, and apnea. Head injury, central nervous system infection, and asphyxia are generally known as leading causes of brain death in infants and children.3 Common causes of brain death in neonates are hypoxic ischemic encephalopathy, birth trauma, cerebral infection, cerebral hemorrhage, malformations, and metabolic diseases.4
Diagnosis of brain death is based on the patient's history and clinical examinations, including the apnea test and loss of brainstem reflexes (light reflex of pupils, gag or sucking reflexes, corneal reflex, and oculovestibular reflex). These examinations should be performed by two different attending physicians. At the time of diagnosis, other possible causes for a comatose state such as hypothermia under 35℃, hypotension, shock, altered metabolic status, or drug intoxication should be excluded. In cases where clinical examination results are uncertain or insufficient, additional ancillary tests (electroencephalogram or radionuclide cerebral blood flow) may be considered.5
An apnea test is implemented to confirm lack of spontaneous respiration when the arterial PaCO2 is elevated over 60 mmHg. This is to prove the absence of respiratory control system reflexes in the brainstem when intense physiologic stimulation to breathe occurs. Recently, the apnea test itself and its utility have been questioned. As in our case, it has been reported that the test had to be discontinued when hemodynamic instability was observed before an appropriate PaCO2 was reached.67 Therefore, a modified apnea test that does not require removal of the conventional mechanical ventilator at once, but instead gradually lowers the effect of the device, has been used in some intensive care units.8
To determine brain death in newborns, neurological imaging studies such as cerebral blood flow may be helpful, especially when the results of the physical examination are unclear or the apnea test is unavailable. Although four-vessel cerebral angiography is an invasive and costly process, it has traditionally been used as a confirmative tool for determination of brain death. In addition, various methods such as radionuclide cerebral blood flow, electroencephalogram, transcranial ultrasonography, and magnetic resonance imaging/angiography have been used to obtain additional information to diagnose brain death.9 However, these further studies require patient transportation to different examination rooms and are often impossible for patients with respiratory and cardiovascular instability.
In Korea, there has been a recent revision of the national law for the diagnostic criteria for brain death in 2013, but it still does not include standard for infants less than 2 months old.10 Due to a lack of specific guidelines for infants younger than 2 months, our NICU's policy for diagnosis of brain death in newborns is not based on a legal standard. To determine brain death, we refer to “An update of the 1987 task force recommendations” revised in 2011 by Nakagawa et al.5 However, this report still lacks a standard for preterm babies. It is especially difficult to confirm brain death in premature babies in the NICU, because evaluation of brainstem function or level of consciousness in preterm babies under 37 weeks of gestational age is an uncertain and challenging process.11 Therefore, it is necessary to establish national standards to declare brain death in neonates, including preterm babies.
What are the appropriate measures to ensure that the infant's best interests are taken into account after the determination of brain death? Major reasons for diagnosing brain death are to permit withholding treatment and to provide organs for transplantation (Organ donation and transplantation are beyond the scope of this article and are not therefore discussed.). With artificial ventilation, the heart continues to beat for a period of time after brain death. Time lag between brain death and circulatory death is usually less than one month in adults.12 However, no study has examined the life expectancy of brain dead newborns on respiratory support; there is only one report of two neonatal cases that survived longer than expected.13 In addition, there are no guidelines for end-of-life treatment, including prolonging life, palliative care, and withholding treatment in a brain dead newborn. Deciding whether to prolong life or withdraw treatment in a brain dead newborn is extremely challenging, even though the newborn will eventually go into cardiac arrest. Withdrawing treatment most commonly takes the form of withdrawal of ventilator support. The decision to prolong life or withhold treatment is not only a medical issue, but also an ethical, legal, cultural, and religious issue, and places a heavy burden on the physicians and parents. The decision to withdraw life-support requires enough communication between the medical staff and the baby's parents. At our institute, we have an ethics committee that we can present putative cases of brain death to. We use this ethics committee to alleviate our ethical and legal burden regarding the decision to withdraw life-support, and follow its rulings. We emphasize the need for guidelines for end-of-life treatment for brain dead newborns at the national level.
In conclusion, we report a case of brain death due to hypoxic ischemic encephalopathy in a near-term newborn. We emphasize the need to establish guidelines for brain death in newborns and further directions regarding end-of-life treatment after brain death, based on national standards.
Figures and Tables
References
1. Parker BL, Frewen TC, Levin SD, Ramsay DA, Young GB, Reid RH, et al. Declaring pediatric brain death: current practice in a Canadian pediatric critical care unit. CMAJ. 1995; 153:909–916.

2. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographics study. Arch Neurol. 1976; 33:696–705.
3. Ashwal S, Serna-Fonseca T. Brain death in infants and children. Crit Care Nurse. 2006; 26:117–124. 126–128.

4. Ashwal S. Brain death in the newborn. Current perspectives. Clin Perinatol. 1997; 24:859–882.
5. Nakagawa TA, Ashwal S, Mathur M, Mysore M. Society of Critical Care Medicine, Section on Critical Care and Section on Neurology of American Academy of Pediatrics. Child Neurology Society. Clinical report-guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations. Pediatrics. 2011; 128:e720–e740.
6. Ding ZY, Zhang Q, Wu JW, Yang ZH, Zhao XQ. A comparison of brain death criteria between China and the United States. Chin Med J. 2015; 128:2896–2901.

7. Gillson N, Weisleder P, Ream MA. A brain death dilemma: apnea testing while on high-frequency oscillatory ventilation. Pediatrics. 2015; 135:e5–e6.

8. Ahlawat A, Carandang R, Heard SO, Muehlschlegel S. The modified apnea test during brain death determination: an alternative in patients with hypoxia. J Intensive Care Med. 2016; 31:66–69.
9. Wahlster S, Wijdicks EF, Patel PV, Greer DM, Hemphill JC 3rd, Carone M, et al. Brain death declaration: Practices and perceptions worldwide. Neurology. 2015; 84:1870–1879.

10. Internal Organs. . Transplant Act of 2013, Article 18 (Act No. 11976).
11. Chatziioannidis I, Chouchou P, Nikolaidis N. Is brain death diagnosis in newborns feasible? Hippokratia. 2012; 16:308–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download