Abstract
Purpose
The aim of this study was to evaluate this clinical outcome of neonatal pelvis dilatation in very low birth weight (VLBW) infants.
Methods
The medical records of 127 VLBW infants admitted to two neonatal intensive care units from January 2012 to December 2014 were retrospectively analyzed. Renal pelvis dilatation was diagnosed via ultrasound examination with cases divided into 3 groups: mild (dilatation of 5-10 mm), moderate (11-15 mm) and severe (≥15 mm). The correlation between the 3 dilatation groups and progression into hydronephrosis was evaluated.
Results
Among the 127 premature infants, renal pelvis dilatation was identified in 29 (22.8%) on ultrasound examination performed, on average 13.3 days after birth, combined with calyceal ectasia in 5 (3.9%) infants. At a postmenstrual age of 40 weeks, 18 infants (14.2%) had renal pelvis dilatation, 6 (5%) infants accompanied by a dilatation of the calyx. On the last follow-up performed when children were not older than 2 years old, renal pelvis dilatation had resolved to within normal limits in 23 (79%) infants while persisting in 6 (21%) infants; 1 mild, 3 moderate, and 2 severe pelvis dilatation. The median time-to-recovery of dilatation was 10.5 months (Kaplan-Meier curve), regardless of the severity of dilatation at birth. Based on receiver operating curve analysis, a cutoff diameter of renal dilatation at birth of 11.35 mm predicted persisting severe dilatation at the final follow-up (sensitivity 83.3%, specificity 82.6%).
Renal pelvis dilatation occurs in 1-2% of fetuses.12 As renal pelvis antero-posterior (AP) diameters vary with gestational age, antenatal diagnosis of hydronephrosis is based on different criteria for each trimester of pregnancy.345 Although the Society for Fetal Urology has published a consensus statement regarding the criteria for antenatal diagnosis of hydronephrosis,6 these criteria have not been globally adopted. Therefore a unified definition and grading of renal pelvis dilatation does not currently exist. As an example postnatal renal pelvis dilatation has been defined using multiple measurement categories, such as a dilatation ≥5 mm or ≥7 mm,78 with other grading systems defining moderate dilatation as an AP diameter >10 mm and severe dilatation as an AP diameter ≥15 mm.91011
Studies have reported an association between renal pelvis dilatation and postnatal progression to other renal diseases, including renal anomalies in 62% of cases of renal pelvis dilatation and significant uropathy in 39% of cases.10 An analysis of the progression to renal disease based on gestational age indicated that 12% of infants showing pelvis dilatation in the second trimester developed uropathy such as renal duplication, vesico-ureteral reflux (VUR) and ureteropelvic junction obstruction (UPJO), while 69% of those showing pelvis dilatation in the third trimester developed renal anomalies. An AP diameter ≥7 mm in the third trimester is a predictive indicator of postnatal uropathy.12
Treatment for renal pelvis dilatation is based on the severity of the condition. Ninety percent of infants with a mild dilatation did not require surgical treatment, while 17% of infants with moderate dilatation and all infants with severe dilatation progressed to obstruction of the pyelo-ureteral junction, which required surgical treatment.1213 Based on this evidence, close follow-up monitoring of infants with severe hydronephrosis for up 2 years was recommended.1314 Others reported that 87% of infants with hydronephrosis recovered with only conservative management.1516 Among Korean infants diagnosed with hydronephrosis on antenatal ultrasound examination, 47% of infants were confirmed ureteropelvic obstruction or vesicoureteral reflux, and only 10% of them were required surgical treatment.17 Therefore renal pelvis dilatation is an important predictive factor of subsequent uropathy requiring surgical treatment that depends on the severity of dilatation.18 However reported outcomes of renal pelvis dilatation at birth have varied widely among different institutions and different studies. Studies to date have either not included premature infants or have not specifically evaluated outcomes relative to prematurity. Therefore the aim of our study was to specifically evaluate the clinical progression of renal pelvis dilatation identified at birth, in very low birth weight (VLBW) infants.
One hundred forty VLBW infants had been treated in the intensive care unit at Severance Hospital and Gangnam Severance Hospital, between January 2012 and December 2014 and 13 expired infants were excluded. All VLBW infants who admitted were performed general blood tests, renal function tests, urine tests and ultrasound. The medical records of 127 VLBW infants who had undergone renal ultrasound examination in the neonatal intensive care unit, were reviewed for this study. Dilatation was defined by AP diameter ≥5 mm on renal ultrasound examination.3 A diameter between 10 mm and 15 mm was defined as a moderate dilatation, with severe dilatation defined as a diameter >15 mm, based on a previously published grading system of fetal pelvis dilatation in the third trimester (Fig. 1).819 Outcomes were evaluated by ultrasound examination performed at a postmenstrual age (PMA) of 40 weeks, with the final follow-up examination performed when children were not older than 2 years old. All ultrasound examinations and renal measurements were performed by staff radiologists in both hospitals. Ethical approval was obtained from the ethics committees of Severance Children's Hospital and Gangnam Severance Hospital.
All statistical analyses were performed using the SPSS software v.23 (IBM Corp., Armonk, NY., USA). A P-value <0.05 was considered statistically significant. Kaplan-Meier curves were used to estimate the recovery from pelvis dilatation using lifetime data. A log-rank test was performed to compare the probability of recovery among the three severity-based groups: mild, moderate and severe. We calculated receiver operating characteristic (ROC) curves to predict the risk of persisting renal pelvis dilatation at last follow-up. We also performed a sensitivity analysis to determine the optimal cutoff points for predicting persistent dilatation using ROC curves.
Our study group of 127 VLBW infants included 60 males and 67 females, with a mean±standard deviation gestational age of 29.2±2.8 weeks (range 24.1 to 36.1), and a birth weight of 1,120.4±237.2 g (range 400 to 1,490) (Table 1). All infants performed prenatal ultrasound and there was only one infant who has renal pelvis dilatation. The initial ultrasound examination was performed, on average, 13.3 days (range 1 to 78 days) after birth, with the last ultrasound examination performed, on average, 6.3 months (range 1 to 24 months) after birth. If the initial ultrasound examination showed abnormal findings, repeated ultrasound examinations were performed up until the point of recovery (i.e., normal findings on examination) or up to 2 years of age. The distribution of findings on ultrasound examinations is summarized in Table 2.
Initial ultrasound examination at birth was unremarkable in 98 (77%) infants, with a renal pelvis dilatation identified in 29 (22%) infants. In terms of the distribution of renal pelvis dilatation, 10 infants presented with bilateral dilatation, 18 with dilatation on the left side, and 1 with a dilatation on the right side. In 5 infants, renal pelvis dilatation was accompanied with a dilated calyx, which was clinically diagnosed as hydronephrosis. Bilateral hydronephrosis was identified in 1 infant and left-sided hydronephrosis in 4 infants. Kidney echogenicity was identified in 21 (17%) infants, nephrocalcinosis was identified in 2 (2%) infants (Table 2).
At PMA 40 weeks, 109 infants (86%) had unremarkable ultrasonography examinations, with renal pelvis dilatation persisting in 18 infants (14%). Among these infants, pelvis dilatation was evident on both sides in 8 and isolated to the left side in 10. Among these 18 infants, renal pelvis dilatation was accompanied by a dilatation of the calyx in 6 infants, 1 with bilateral dilatation of the calyx and 5 with left-sided dilatation. With regards to the severity, renal pelvis dilatation was considered to be mild in 10 infants, moderate in 5 and severe in 3. Kidney echogenicity increased in 8 (6%) infants and nephrocalcinosis was identified in 2 (2%) infants. For the remaining 12 infants with pelvis dilatation only, the extent of dilatation showed decreasing trends at the PMA 40 week (Table 2).
Over our follow-up period, the renal pelvis dilatation resolved to within normal limits in 23 (79%) infants, while persisting in 6 infants, 3 with bilateral dilation and 3 with a dilatation only on the left side. Recovery from a renal pelvis dilation of moderate severity was identified in 5 patients (from 8 infants at birth to 3 infants at the last follow-up) and from a severe dilatation was found in 3 patients (from 5 infants at birth to 2 infants at the last follow-up). Therefore, recovery from a severe dilatation of the renal pelvis may be possible but is likely to occur over a longer period. Renal cortical echogenicity increased in 6 (5%) infants, with nephrocalcinosis identified in 2 (2%) infants (Table 2).
Clinical progress of the infants with renal pelvis dilatation is shown in Fig. 2. The number of infants diagnosed with renal pelvis dilation only decreased from 24 to 3, while the number of infants with pelvis dilatation accompanied by dilatation of the calyx decreased from 5 to 3. An overall incidence rate of urinary tract infection of 12% was identified, including 5 infants with pelvis dilatation identified on initial ultrasound examination. Identified pathogens were as follows; Enterococcus (47%), Escherichia coli (18%), Enterobacter (12%), Coagulase negative staphylococci (6%), Klebsiella (6%), and Candida (6%). Therefore, the specific incidence rate of urinary tract infection among infants with an identified renal pelvis dilation at birth was 17%, indicating that pelvis dilatation was not a specific risk factor for urinary tract infection. Among infants with pelvis dilatation and those with hydronephrosis, 63% and 40% were males, respectively.
The recovery of renal pelvis dilatation based on lifetime data using Kaplan Meier curves was estimated to occur at a median age of 10.5 months (range 1-18 months; 25th percentile: 5.1 months; 75th percentile: 15.1 months; Fig. 3). When the VLBW infants with pelvis dilatation at birth were divided into three groups (mild, moderate and severe), no significant between-group differences using log rank test in the rate of recovery were identified (Fig. 3).
ROC curves are shown in Fig. 4. The AUC for persisting renal pelvis dilatation at the last follow-up was 0.88 (P=0.005). The estimated optimal cutoff points to predict remaining pelvis dilatation at last follow up was an AP diameter of 11.35 mm (sensitivity 83.3%, specificity 82.6%).
Renal pelvis dilatation is an important predictive factor of subsequent nephronuropathy. Although several studies have reported on the clinical progression of infantile renal pelvis dilatation20, the clinical progression in VLBW premature infants has not been previously evaluated. We found that most infants with pelvis dilatation showed recovery and that a dilatation ≥11.35 mm was a risk factor for persisting pelvis dilatation.
Lyn et al. measured the AP diameter in 180 fetuses to determine the 10th, 50th, and 90th percentile values by gestational age. The AP diameter increased in direct proportion to gestational age, with a 90th percentile value of 5.0 mm in the second trimester, compared to 50th and 90th percentile values of 4.0 mm and 7.0 mm in the third trimester.21 The mean gestational age of VLBW infants enrolled in our study was 29.2 weeks and, therefore, we graded the severity of renal pelvis dilatation based on measurements for the third trimester. Due to many study variation, researchers and clinicians have argued that a renal pelvis dilatation >5 mm, regardless of gestational age, is a predictive factor for postnatal nephrouropathies, with these infants requiring close follow-up and medical care.3 Therefore, in our study, we considered an AP diameter >5 mm to be an abnormal finding. Based on a previously published grading of antenatal renal pelvis dilatation in the third trimester,819 we defined a dilatation of 5-10 mm as being of mild severity, with diameters of 10-15 mm and >15 mm as moderate and severe dilatations, respectively.
Fetal pelvis dilatation is the most common anomaly detected on prenatal mid-trimester ultrasound. Male fetuses exhibit a significantly higher frequency of renal pelvis dilatation than female fetuses, possibly owing a high rate of spontaneous resolution. Khalid reported an overall incidence rate of pelvis dilatation of 4.5% in fetuses.10 The higher prevalence of renal pelvis dilatation among premature infants, compared to all other infants born at term, might be suggested; however the frequency in preterm infants has not been reported yet. In this study, 19% of VLBW infants presented with pelvis dilatation, and persisting pelvis dilatation through the last follow-up was observed in 5% of cases.
The fact that renal pelvis dilatation is a good prognostic factor for subsequent renal diseases has been confirmed in a number of previously published studies.2223 Khalid reported that 69% of infants with pelvis dilatation in the third trimester on fetal ultrasound were diagnosed with a renal anomaly postnatally.1024 Bouzada conducted a follow-up study of 103 newborn infants with an AP diameter ≥5 mm, providing evidence of the sensitivity and specificity of diameters of 7.5 mm and 15 mm as predictive factors of uropathy and the need for surgical interventions, respectively.12 In our retrospective study, among infants diagnosed with severe renal pelvis dilatation (>15 mm), none progressed to uropathy or required surgical intervention during our follow-up period. Therefore, we could not validate the usefulness of pelvis dilatation in VLBW infants as a predictive factor for postnatal uropathy. However, an AP diameter ≥11.35 mm was predictive of a persisting dilatation at the last follow-up, with a sensitivity of 83.3% and a specificity of 82.6%.
Among the 29 infants in our study with a renal pelvis dilatation diagnosed at the initial renal ultrasound examination, 23 (79.3%) infants recovered normal renal diameters over the follow-up period. However, 88% of infants diagnosed with renal pelvis dilation only showed the improvement, while 40% of infants with pelvis dilatation accompanied by dilatation of the calyx showed the improvement. It means that renal pelvis dilatation accompanied by calyceal dilatation is more difficult to recover than renal pelvis dilatation only. Moreover, the remaining 6 infants did not require surgical intervention over the follow-up period.
We recognize that previous studies evaluating the long-term clinical outcomes of infantile renal pelvis dilatation have reported various outcomes regarding uropathy.In case series of 19 infants with severe hydronephrosis (G3, 4), Onen reported the need for surgical intervention in 35% of cases.14 In contrast, in a case series of 16 infants with moderate-to-severe bilateral hydronephrosis, Bajpai reported that 78% recovered without treatment, with 12.5% requiring unilateral pyeloplasty.16 Another study reported that 87% of infants with hydronephrosis and obstruction of the ureteropelvic junction recovered with conservative management.1516 Based on previous data, close follow-up of infants with severe hydronephrosis for up 2 years is recommended.1314 In this study, the recovery of renal pelvis dilatation was estimated at a median age of 10.5 months (range 1-18 months), with the time to recovery being independent of the severity of the dilatation at initial assessment.
In the interpretation of our results, it is important to note that our study group was small and that the follow-up was conducted over a limited time period. As further surgical intervention may occur after infancy, a long-term follow-up is necessary.
In conclusion, although premature infants with dilatation of mild severity are expected to recover, those with a moderate-to-severe dilatation are at risk of progressing to hydronephrosis. But there was no significant difference among the groups of severity in the rate of recovery. And the estimated optimal cutoff points to predict remaining pelvis dilatation at last follow up was an AP diameter of 11.35 mm (sensitivity 83.3%, specificity 82.6%). Based on current evidence, we recommend that kidney evaluation by ultrasonography should be continued until confirmation of recovery of the dilatation.
Figures and Tables
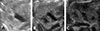 | Fig. 1Ultrasound findings of pelvis dilatation. (A) severe pelvis dilatation (B) moderate pelvis dilatation (C) mild pelvis dilatation. |
 | Fig. 2Clinical progress of the infants with renal pelvis dilatation.Abbreviation: PMA, postmenstural age.
|
 | Fig. 3Days to the recovery of renal pelvis dilatation in VLBW infants using Kaplan Meier curves. Quartile estimates according to the time variable duration showed as follows; 25 percentile of the recovery of renal pelvis dilatation occurred after the 5 months from birth, 50% percentile after 10.5 months from birth, and 75 percentile after 15.1 month from birth. No significant differences in the rate of recovery using log rank test were identified among three groups (mild, moderate and severe). |
 | Fig. 4Diagnostic accuracy of AP diameter for the diagnosis of severe pelvic dilatation. Area under the curve (AUC) values were obtained from receiver operating characteristic (ROC) curve analysis. |
Table 1
Demographic Characteristics of Very Low Birth Weight Infants
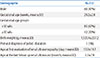
Table 2
Renal Ultrasonography Findings from Birth to Last Follow-up

*Data as absolute numbers; percentages in parentheses.
‡Severity is defined by an AP diameter of renal pelvis. AP diameter of 5-10 mm is defined as mild severity, and diameters of 10-15 mm and >15 mm as moderate and severe dilatations, respectively.
†Last follow-up performed when children were not older than 2 years old.
Abbreviation: PMA, postmenstural age.
References
1. Nguyen HT, Benson CB, Bromley B, Campbell JB, Chow J, Coleman B, et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J Pediatr Urol. 2014; 10:982–998.

2. Mami C, Paolata A, Palmara A, Marrone T, Berte LF, Marseglia L, et al. Outcome and management of isolated moderate renal pelvis dilatation detected at postnatal screening. Pediatr Nephrol. 2009; 24:2005–2008.

3. Scott JE, Wright B, Wilson G, Pearson IA, Matthews JN, Rose PG. Measuring the fetal kidney with ultrasonography. Br J Urol. 1995; 76:769–774.

4. Gunn TR, Mora JD, Pease P. Antenatal diagnosis of urinary tract abnormalities by ultrasonography after 28 weeks' gestation: incidence and outcome. Am J Obstet Gynecol. 1995; 172:479–486.

5. Herndon CD. Antenatal hydronephrosis: differential diagnosis, evaluation, and treatment options. ScientificWorldJournal. 2006; 6:2345–2365.

6. Nguyen HT, Herndon CD, Cooper C, Gatti J, Kirsch A, Kokorowski P, et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol. 2010; 6:212–231.

7. Duncan KA. Antenatal renal pelvic dilatation; the long-term outlook. Clin Radiol. 2007; 62:134–139.

8. Hothi DK, Wade AS, Gilbert R, Winyard PJ. Mild fetal renal pelvis dilatation: much ado about nothing? Clin J Am Soc Nephrol. 2009; 4:168–177.
9. Grignon A, Filion R, Filiatrault D, Robitaille P, Homsy Y, Boutin H, et al. Urinary tract dilatation in utero: classification and clinical applications. Radiology. 1986; 160:645–647.

10. Ismaili K, Hall M, Donner C, Thomas D, Vermeylen D, Avni FE, et al. Results of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am J Obstet Gynecol. 2003; 188:242–246.

11. Cheng AM, Phan V, Geary DF, Rosenblum ND. Outcome of isolated antenatal hydronephrosis. Arch Pediatr Adolesc Med. 2004; 158:38–40.

12. Bouzada MC, Oliveira EA, Pereira AK, Leite HV, Rodrigues AM, Fagundes LA, et al. Diagnostic accuracy of fetal renal pelvis anteroposterior diameter as a predictor of uropathy: a prospective study. Ultrasound Obstet Gynecol. 2004; 24:745–749.

13. Masson P, De Luca G, Tapia N, Le Pommelet C, Es Sathi A, Touati K, et al. Postnatal investigation and outcome of isolated fetal renal pelvis dilatation. Arch Pediatr. 2009; 16:1103–1110.
14. Onen A, Jayanthi VR, Koff SA. Long-term followup of prenatally detected severe bilateral newborn hydronephrosis initially managed nonoperatively. J Urol. 2002; 168:1118–1120.

15. Karnak I, Woo LL, Shah SN, Sirajuddin A, Ross JH. Results of a practical protocol for management of prenatally detected hydronephrosis due to ureteropelvic junction obstruction. Pediatr Surg Int. 2009; 25:61–67.

16. Bajpai M, Chandrasekharam VV. Nonoperative management of neonatal moderate to severe bilateral hydronephrosis. J Urol. 2002; 167:662–665.

17. Park YJ, Mun SJ, Bae CW, Lee BH, Kim JI. Post-natal outcome of fetal hydronephrosis detected with prenatal ultrasonography. J Korean Pediatr Soc. 2002; 45:1213–1218.
18. Chen SY, Su YT, Wu CY. Nonobstructive dilation of urinary tract and later development of obstruction: report of one case. Pediatr Neonatol. 2010; 51:353–355.

19. Odibo AO, Marchiano D, Quinones JN, Riesch D, Egan JF, Macones GA. Mild pyelectasis: evaluating the relationship between gestational age and renal pelvic anterior-posterior diameter. Prenat Diagn. 2003; 23:824–827.

20. Policiano C, Djokovic D, Carvalho R, Monteiro C, Melo MA, Graça LM. Ultrasound antenatal detection of urinary tract anomalies in the last decade: outcome and prognosis. J Matern Fetal Neonatal Med. 2015; 28:959–963.

21. Chitty LS, Altman DG. Charts of fetal size: kidney and renal pelvis measurements. Prenat Diagn. 2003; 23:891–897.

23. Arena S, Magno C, Montalto AS, Russo T, Mami C, Baldari S, et al. Long-term follow-up of neonatally diagnosed primary megaureter: rate and predictors of spontaneous resolution. Scand J Urol Nephrol. 2012; 46:201–207.

24. Ismaili K, Avni FE, Wissing KM, Hall M. Brussels Free University Perinatal Nephrology Study. Group Long-term clinical outcome of infants with mild and moderate fetal pyelectasis: validation of neonatal ultrasound as a screening tool to detect significant nephrouropathies. J Pediatr. 2004; 144:759–765.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download