1. Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012; 129:1019–1026.

2. Kim KS, Bae CW. Trends in survival rate for very low birth weight infants and extremely low birth weight infants in Korea, 1967-2007. Korean J Pediatr. 2008; 51:237–242.

3. Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009; 124:717–728.

4. Shankaran S, Johnson Y, Langer JC, Vohr BR, Fanaroff AA, Wright LL, et al. Outcome of extremely-low-birth-weight infants at highest risk: gesational age < or =24 weeks, birth weight < or =750 g, and 1-minute Apgar < or =3. Am J Obstet Gynecol. 2004; 191:1084–1091.
5. Anderson PJ, Cheong JL, Thompson DK. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin Perinatol. 2015; 39:147–158.

6. de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA. 2009; 302:2235–2242.
7. Marlow N, Wolke D, Bracewell MA, Samara M. Group EPS. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005; 352:9–19.

8. Larroque B, Ancel PY, Marret S, Marchand L, Andre M, Arnaud C, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008; 371:813–820.

9. Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF, Vermont Oxford. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology. 2010; 97:329–338.

10. Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. 2014; 19:90–96.

11. Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006; 355:685–694.

12. Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005; 147:609–616.

13. Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003; 143:171–179.

14. Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One. 2012; 7:e51879.

15. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004; 292:2357–2365.

16. Perlman JM, Risser R, Broyles RS. Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics. 1996; 97:822–827.

17. Volpe JJ. Postnatal sepsis, necrotizing enterocolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr. 2008; 153:160–163.
18. Nongena P, Ederies A, Azzopardi DV, Edwards AD. Confidence in the prediction of neurodevelopmental outcome by cranial ultrasound and MRI in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2010; 95:F388–F390.

19. Setänen S, Haataja L, Parkkola R, Lind A, Lehtonen L. Predictive value of neonatal brain MRI on the neurodevelopmental outcome of preterm infants by 5 years of age. Acta Paediatr. 2013; 102:492–497.
20. El-Dib M, Massaro AN, Bulas D, Aly H. Neuroimaging and neurodevelopmental outcome of premature infants. Am J Perinatol. 2010; 27:803–818.

21. Kwon SH, Vasung L, Ment LR, Huppi PS. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin Perinatol. 2014; 41:257–283.

22. Mirmiran M, Barnes PD, Keller K, Constantinou JC, Fleisher BE, Hintz SR, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics. 2004; 114:992–998.

23. Horsch S, Skiöld B, Hallberg B, Nordell B, Nordell A, Mosskin M, et al. Cranial ultrasound and MRI at term age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2010; 95:F310–F314.

24. Maalouf EF, Duggan PJ, Counsell SJ, Rutherford MA, Cowan F, Azzopardi D, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001; 107:719–727.
25. Skiöld B, Vollmer B, Böhm B, Hallberg B, Horsch S, Mosskin M, et al. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J Pediatr. 2012; 160:559–566.

26. Van't Hooft J, van der Lee JH, Opmeer BC, Aarnoudse-Moens CS, Leenders AG, Mol BW, et al. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst Rev. 2015; 4:71.
27. Brown NC, Inder TE, Bear MJ, Hunt RW, Anderson PJ, Doyle LW. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr. 2009; 155:32–38.

28. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–1729.

29. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33:179–201.

30. Bayley N. Manual for the Bayley Scales of Infant Development. 2nd ed. San Antonio (TX): The Psychological Corporation;1993.
31. Wolke D, Sohne B, Ohrt B, Riegel K. Follow-up of preterm children: important to document dropouts. Lancet. 1995; 345:447.

32. Callanan C, Doyle L, Rickards A, Kelly E, Ford G, Davis N. Children followed with difficulty: how do they differ? J Paediatr Child Health. 2001; 37:152–156.

33. Castro L, Yolton K, Haberman B, Roberto N, Hansen NI, Ambalavanan N, et al. Bias in reported neurodevelopmental outcomes among extremely low birth weight survivors. Pediatrics. 2004; 114:404–410.

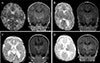

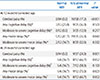
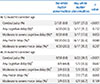
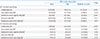




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download