Abstract
Pancreatic lymphoepithelial cysts (LECs) are rare pancreatic cysts with squamous epithelial lining surrounded by dense lymphoid tissue. A preoperative diagnosis of LECs is difficult due to imaging diversity and inadequate documentation because of their rarity. We present a case of surgically confirmed pancreatic LEC with magnetic resonance imaging (MRI) findings as heterogeneous signal intensity on T2-weighted images with multiple septa-like structures, slightly hypo-signal intensity on T1-weighted images, and thin-wall enhancement on dynamic contrast images. LECs are benign lesions without any malignant potential. Therefore, the inclusion of LEC in the differential diagnosis of cystic pancreatic lesions may reduce unnecessary surgical procedures.
Lymphoepithelial cysts (LECs) of the pancreas are very rare, benign, and non-malignant lesions, which were first described in 1985 by Lüchtrath and Schriefers (1). Kavuturu et al. (2) reported that in the 28 years since the first report on LEC, only 109 cases have so far been documented in the literature. LECs are true pancreatic cysts filled with keratinized material, lined by keratinizing squamous epithelium, and surrounded by lymphoid tissue (3). Recent reviews suggest, that LECs are predominantly seen in males, anywhere in the pancreas with variable size. About half of the patients are asymptomatic and receive clinical attention based on incidental radiological findings, while the remaining patients present with non-specific symptoms such as nausea, vomiting, diarrhea, abdominal pain, weight loss, or fatigue.
Because of the benign nature of LECs without any malignant potential, accurate identification is important to avoid unnecessary intervention. However, it is difficult to differentiate the LECs from other solid and cystic pancreatic lesions on preoperative imaging because the imaging appearances vary from patient to patient (2). Furthermore, studies describing imaging features of LECs are limited to case reports or small case series, and those including magnetic resonance imaging (MRI) findings are even scarcer. Herein, we report a case of surgically confirmed LEC of the pancreas based on the MRI findings.
A 59-year-old previously healthy man presented with a 1-year history of right upper abdominal pain. He had no history of smoking or alcohol abuse. Physical examination was unremarkable. Contrast-enhanced computed tomography (CT) demonstrated 1 cm exophytic well-defined oval lesion with thin peripheral wall enhancement in the pancreatic tail portion. The pancreas showed normal contour and attenuation without any abnormal ductal dilatation on CT. Endoscopic ultrasound (EUS) revealed a 1 cm well-encapsulated hypoechoic mass-like lesion with multiple internal anechoic lesions in the correlated area. EUS-guided fine needle aspiration (FNA) was not performed because of its risk considering the relatively small size of the lesion and its location at the pancreatic tail portion. A follow-up CT examination performed 7 months later showed an increase in the size of the lesion from 1 cm to 1.3 cm (Fig. 1a, b). Blood tests performed during the same period revealed that amylase and lipase levels, as well as carbohydrate antigen (CA) 19-9, were within the normal range.
For further evaluation, the patient underwent abdominal MRI (Ingenia 3T, Philips Healthcare, Best, the Netherlands) with gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA). The lesion showed heterogeneous signal intensity on T2-weighted MRI with fairly honeycomb-like appearance containing multiple septa-like structures inside (Fig. 1c). The lesion showed slightly hypo-signal intensity on precontrast T1-weighted image compared to the normal pancreatic parenchyma, and thin peripheral wall enhancement on dynamic contrast enhancement images (Fig. 1d-g). In diffusion-weighted imaging (DWI), the lesion showed hyperintensity sustained with increased b values (0, 20, 400, and 800 s/mm2). However, the apparent diffusion coefficient (ADC) values of the lesion were similar to those of the remaining pancreatic parenchyma (Fig. 1h, i). There was no area of significant signal drop at the in- and out-of-phase chemical shift imaging. Magnetic resonance cholangiopancreatography (MRCP) revealed no evidence of abnormal ductal dilatation of pancreas or ductal communication with the lesion. Thus, our differential diagnosis of the lesion included cystic neuroendocrine tumor and mucinous or serous cystic tumor. Distal pancreatectomy revealed a cystic nodule measuring 1.3 cm in size with the pale yellowish surface. The nodule was encapsulated by a fibrous capsule, filled with keratin material, and was pathologically confirmed as a LEC (Fig. 1j).
LECs of the pancreas are extremely rare and benign pancreatic cysts accounting for approximately 0.5% of all pancreatic cysts (4). Because of its rarity, the lesion is poorly characterized clinically, radiologically, and pathologically. Several etiological theories defining LECs have been suggested, including squamous metaplasia of pancreatic duct, epithelial remnants in lymph node, displacement of branchial cysts that fuse with pancreas during embryogenesis, or a possible teratoma (4). Conditions often associated with LECs in salivary gland such as Sjogren's disease, HIV infection, and lymphoma have not been documented in pancreatic LECs. Even though the exact etiology remains unclear, LECs in the pancreas are differentiated histologically from other types of well-established disease entities (5).
LECs are usually round and have a well-defined wall that is sharply demarcated from the pancreas and surrounding adipose tissue. They often protrude from the pancreatic parenchyma and tend to be peripancreatic as in our case. No preferential location of LECs has been determined in the pancreas. In a study of 12 cases reported by Adsay et al. (4), the mean size of LECs was 4.6 cm. Morphologically, they were either multilocular or unilocular with macrocystic appearance. Cysts are typically filled with keratinized materials displaying “cheesy” or “caseous” appearance on macroscopic examination. However, in some cases, the contents of the cyst may be clear and serous, which may lead to confusion regarding differentiation of LECs from serous cystadenomas. Microscopically, LECs are lined by mature stratified squamous epithelium and surrounded by subepithelial lymphoid tissue containing lymphoid follicles. The LECs contain acanthotic projections into the surrounding lymphatic tissue and are separated from the pancreatic parenchyma by a capsule of the thin fibrotic capsule (3).
On CT, LECs appear as a multicystic mass or as a cyst with thin septa-like structures in most of the cases (6). Generally, small solid components or papillary projections, thin-walled enhancements or wall calcifications are variably seen. On MRI, the cystic nature of LECs is well reflected as hypointense on T1-weighted images and hyperintense on T2-weighted images. However, LECs may be complex with cholesterol clefts in the keratin content, thus altering the signal intensities on T1- and T2- weighted images. Motion restriction of water molecules may be seen on DWI due to internal keratinized material similar to other squamous-lined cysts present elsewhere (7). Signal loss may be observed on out-of-phase gradient-echo images due to the presence of intracellular lipids (8). There is usually no communication with a pancreatic duct on MRCP.
In our case, multiple septa-like structures present inside the lesion were clearly depicted on T2-weighted images with high signal intensity. These septa-like structures showed no enhancement and were not evident on dynamic contrast enhancement, probably because they are actually cholesterol clefts interposed between the keratin components. Considering that the septum in other cystic tumors is a true septum, which usually shows low-signal intensity on the T2-weighted image, these septa-like structures may be an MRI feature characteristic of LECs. These structures may differentiate LECs from other cystic tumors with septa such as serous or mucinous cystic neoplasms and intraductal papillary mucinous neoplasms. In the present case, thin peripheral wall enhancement observed on the dynamic contrast enhancement images was strongly correlated with the thin fibrotic capsule observed in the microscopic examination. Although the lesion was filled with keratin material, restriction of water molecules on DWI was not evident probably due to the relatively small size of the lesion.
Clinical differentiation of LECs from other pancreatic lesions could be a challenging issue. Traditional markers such as carcinoembryonic antigen (CEA), CA 19-9, and CA 72-4 are expected to be lower in LECs compared to mucinous neoplasms, which is not confirmed due to limited experience. EUS with FNA cytopathology might be of help in diagnosing LECs (9), but elevated CEA and CA 19-9 levels have been reported in fluid aspirated from confirmed LECs, leading to diagnostic confusion (10). LECs are benign lesions, and therefore, when a pancreatic lesion is highly suspected to be a LEC, invasive surgical intervention is undesirable and may be followed up with serial cross-sectional abdominal imaging. However, surgical intervention is inevitable in most of the cases owing to the radiological and clinical features mimicking the features of other solid and cystic tumors of the pancreas.
In conclusion, we report a case of small pancreatic LEC with MRI showing heterogeneous signal intensity on T2-weighted images with multiple septa-like structures, slightly hypo-signal intensity on T1-weighted images, and thin-wall enhancement on dynamic contrast images. It is proposed that the MRI features may facilitate the differential diagnosis of cystic pancreatic lesions despite the rarity of LECs.
Figures and Tables
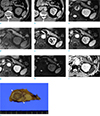 | Fig. 1A lymphoepithelial cyst of the pancreas in a 59-year-old man. A 1.3-cm-sized well-defined oval lesion is seen in the pancreatic tail portion with an exophytic appearance. The lesion shows isoattenuation with pancreas on precontrast CT scan (a) and thin peripheral wall enhancement on postcontrast CT scan (b). On T2-weighted image (c), the lesion shows heterogeneous signal intensity with multiple septa-like structures present inside. The lesion shows slightly hypo-signal intensity on the precontrast T1-weighted image (d) with thin peripheral wall enhancement on arterial (e), portal (f), and 3-min delayed phase (g) images of a dynamic contrast enhancement study. The lesion shows high signal intensity on axial high b-value image (b = 800 s/mm2) (h); however, the ADC map (i) reveals no diffusion restriction. Photograph of the gross surgical specimen (j) demonstrates cystic nodule with the pale yellowish surface, filled with keratin material, and encapsulated by a fibrous capsule. |
References
1. Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004; 351:1218–1226.

2. Kavuturu S, Sarwani NE, Ruggeiro FM, et al. Lymphoepithelial cysts of the pancreas. Can preoperative imaging distinguish this benign lesion from malignant or pre-malignant cystic pancreatic lesions? JOP. 2013; 14:250–225.
4. Adsay NV, Hasteh F, Cheng JD, et al. Lymphoepithelial cysts of the pancreas: a report of 12 cases and a review of the literature. Mod Pathol. 2002; 15:492–501.

5. Nam SJ, Hwang HK, Kim H, et al. Lymphoepithelial cysts in the pancreas: MRI of two cases with emphasis of diffusion-weighted imaging characteristics. J Magn Reson Imaging. 2010; 32:692–696.

6. Kim YH, Auh YH, Kim KW, Lee MG, Kim KS, Park SY. Lymphoepithelial cysts of the pancreas: CT and sonographic findings. Abdom Imaging. 1998; 23:185–187.

7. Chen S, Ikawa F, Kurisu K, Arita K, Takaba J, Kanou Y. Quantitative MR evaluation of intracranial epidermoid tumors by fast fluid-attenuated inversion recovery imaging and echo-planar diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001; 22:1089–1096.
8. Fukukura Y, Inoue H, Miyazono N, et al. Lymphoepithelial cysts of the pancreas: demonstration of lipid component using CT and MRI. J Comput Assist Tomogr. 1998; 22:311–313.
9. Ahlawat SK. Lymphoepithelial cyst of pancreas. Role of endoscopic ultrasound guided fine needle aspiration. JOP. 2008; 9:230–234.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download