Abstract
Therapeutic hypothermia in cardiac arrest patients is associated with favorable outcomes mediated via neuroprotective mechanisms. We report a rare case of a 32-year-old male who demonstrated complete recovery of signal changes on perfusion-weighted imaging after therapeutic hypothermia due to cardiac arrest. Brain MRI with perfusion-weighted imaging, performed three days after ending the hypothermia therapy, showed a marked decrease in relative cerebral blood flow (rCBF) and delay in mean transit time (MTT) in the bilateral basal ganglia, thalami, brain stem, cerebellum, occipitoparietal cortex, and frontotemporal cortex. However, no cerebral ischemia was not noted on diffusion-weighted imaging (DWI) or fluid-attenuated inversion recovery (FLAIR) sequences. A follow-up brain MRI after one week showed complete resolution of the perfusion deficit and the patient was discharged without any neurologic sequelae. The mechanism and interpretation of the perfusion changes in cardiac arrest patients treated with therapeutic hypothermia are discussed.
In out-of-hospital cardiac arrest patients, therapeutic hypothermia is the optimal strategy to promote patient survival by neuroprotective mechanisms (1). Brain injuries detected on diffusion-weighted imaging (DWI) are known to be predictors of poor clinical outcome (2). However, few studies have reported the findings from perfusion-weighted MRI (PWI) in cardiac arrest patients treated with hypothermia, demonstrating that increased relative cerebral blood flow (rCBF) observed on PWI was associated with unfavorable results (3). However, to the best of our knowledge, studies have yet to report the decrease in rCBF on PWI associated with complete recovery and favorable prognosis on follow-up.
We introduce a case of altered cerebral perfusion in a patient following hypothermia treatment showing complete resolution during follow-up imaging. The mechanism and interpretation of the perfusion changes in cardiac arrest patients following therapeutic hypothermia are investigated.
A 32-year-old male without known past history was transferred to our emergency center. The patient had an initial ventricular fibrillation rhythm and was treated with 20 mins of cardiopulmonary resuscitation. The mental status indicated semi-coma with a Glasgow Coma Scale score of 4. The initial CT did not show any abnormal findings. The patient was admitted to the intensive care unit for post-resuscitation care, including hypothermia therapy. His body temperature was decreased from 35.8℃ to 33.0℃, and after 24 hrs of maintenance, gradual re-warming was performed for 17 hrs (0.2℃/hour). Three days after ending the hypothermia therapy, brain MRI was performed. MRI scans were obtained using a 1.5-T device (Avanto Syngo, Siemens, Erlargen, Germany). The MRI protocol included conventional fluid-attenuated inversion recovery (FLAIR) imaging, DWI, gradient-echo imaging, perfusion imaging, contrast-enhanced cervical MR angiography and intracranial time-of-flight MR angiography. The scan protocol included: T2-weighted axial imaging, FLAIR sequences, T1-weighted axial imaging, and MR angiography with a slice thickness of 5 mm. PWI was acquired by dynamic susceptibility contrast MRI with a repitition time (TR)/echo time (TE) of 1790/33 ms, 20 slices, and 0.1 mmol/kg gadolinium-based contrast medium injection at 4 mL/s. Arterial input function was identified by anterior cerebral artery. The following hemodynamic parameters were observed noninvasively; regional time to peak, rCBF, and mean transit time (MTT). MRI software automatically generated the perfusion maps. PWI showed a marked delay of MTT and reduction of rCBF in bilateral basal ganglia, thalami, brain stem, cerebellum, occipitoparietal cortex, and frontotemporal cortex (Fig. 1a-d). However, no abnormal finding related to ischemia was noted on DWI or apparent diffusion coefficient (ADC) maps (Fig. 2a, b). FLAIR imaging revealed multifocal sulcal hyperintensities, probably related to high-concentration oxygen inhalation (Fig. 2c). To evaluate delayed hypoxic brain injury, a follow-up brain MRI was performed 1 week later, resulting in complete recovery of the previous perfusional abnormalities without any additional abnormal finding on MRI (Fig. 1e-h, Fig. 2d-e). The sulcal hyperintensities on FLAIR imaging also disappeared (Fig. 2f).
After the insertion of implantable cardioverter-defibrillator for ventricular fibrillation, the patient was discharged with alert mental status (Glasgow Coma Scale score 15). No neurological sequelae were detected at the time of discharge.
Diffusion weighted magnetic resonance imaging of brain is used for the evaluation of hypoxic brain injury in patients with cardiac arrest. After cerebral hypoxia, impaired function of ion pumps in cell membrane results in cytotoxic edema, which is reflected by the low ADC values (4). Reduced ADC values are correlated with poor neurological outcome in cardiac arrest patients (25). However, only a few studies reported changes involving PWI in patients with cardiac arrest. A previous study showed that the markedly increased perfusion after cardiac arrest was associated with acute diffusion restrictions and was related to poor prognosis, and ultimately death (3). The findings were probably attributed to the failure of cerebral autoregulation resulting in reactive hyperemia. However, a canine model showed that cardiac arrest followed by resuscitation induced a microcirculation injury, showing hypoperfusion on PWI (significantly decreased rCBF) on the day after resuscitation and subsequent complete recovery of rCBF on follow-up (4). These discrepant findings on PWI might be related to opposite clinical outcomes. We speculate that resuscitated patients with good prognosis show decreased perfusion due to transient microcirculation injury, while patients with poor prognosis show increased perfusion due to hyperemia associated with disruption of cerebral autoregulation. The effect of hypothermia on PWI in patients with cardiac arrest should also be considered. In patients with severe cerebral infarction, hypothermia treatment led to a favorable prognosis showing variable CBF parameters approximately 3 days after the onset of therapeutic hypothermia, which either resulted in complete recovery or decrease from the baseline CBF although the parameter was in recovery phase (6). Hypothermia induces a decrease in cerebral metabolic rate of oxygen, resulting in CBF reduction. Our patient showed decreased perfusion 3 days after hypothermia therapy (6 days after cardiac arrest) and showed complete resolution 10 days later. These findings may suggest an overlapping result due to microcirculation injury and hypothermia. However, further well-designed studies are needed for validation. In our case, there was a marked delay of MTT and reduction of rCBF in bilateral basal ganglia, thalami, brain stem, cerebellum, occipitoparietal cortex, and frontotemporal cortex. These areas represent common sites of brain ischemia vulnerable to excitotoxic injury and high energy demands (7).
Thus, in resuscitated patients, decreased rCBF on PWI without ischemic changes on DWI or ADC maps within three days after ending therapeutic hypothermia may indicate a favorable prognosis. Follow-up brain MRI may be used to assess the recovery of cerebral perfusion. In our case, we performed dynamic susceptibility contrast PWI, which may be limited by the relative perfusion values. Further prospective studies with large sample size should be performed to evaluate the utility of PWI in patient's prognosis.
In conclusion, we report a case of cardiac arrest treated with hypothermia, resulting in complete recovery of abnormalities on PWI. The patient was discharged without any neurologic sequelae. The perfusion changes in the absence of DWI abnormality may indicate favorable prognosis, rather than a dismal one.
Figures and Tables
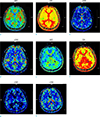 | Fig. 1Initial brain perfusion-weighted imaging (a-d) showed delay in mean transit time (MTT) with decreased relative cerebral blood flow (rCBF) in bilateral basal ganglia, thalami, occipital cortex and temporal cortex (arrows in a, c), which were recovered on follow-up imaging (e-h). rCBV = relative cerebral blood volume; TTP = time to peak |
References
1. Holzer M, Bernard SA, Hachimi-Idrissi S, et al. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005; 33:414–418.

2. Li F, Liu KF, Silva MD, et al. Acute postischemic renormalization of the apparent diffusion coefficient of water is not associated with reversal of astrocytic swelling and neuronal shrinkage in rats. AJNR Am J Neuroradiol. 2002; 23:180–188.
3. Jarnum H, Knutsson L, Rundgren M, et al. Diffusion and perfusion MRI of the brain in comatose patients treated with mild hypothermia after cardiac arrest: a prospective observational study. Resuscitation. 2009; 80:425–430.

4. Liu R, Li X, Hu CL, et al. The changes of brain water diffusion and blood flow on diffusion-weighted and perfusion-weighted imaging in a canine model of cardiac arrest. Resuscitation. 2012; 83:645–651.

5. Youn CS, Park KN, Kim JY, et al. Repeated diffusion weighted imaging in comatose cardiac arrest patients with therapeutic hypothermia. Resuscitation. 2015; 96:1–8.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download