Abstract
Progressive transformation of germinal centers (PTGC) is an atypical feature seen in lymph nodes with unknown pathogenesis. PTGC most commonly presents in adolescent and young adult males as solitary painless lymphadenopathy with various durations. Cervical nodes are the most commonly involved ones while involvements of axillary and inguinal nodes are less frequent. PTGC develops extremely rarely in other locations. We report a rare case of solitary mass present in the presacral space. The mass as subsequently proven to be PTGC. To the best of our knowledge, PTGC in the presacral space has not been previously reported in the literature.
Progressive transformation of germinal centers (PTGC) was initially reported by Lennert and Muller-Hermelink (1). PTGC is an atypical but benign condition of unknown pathogenesis in lymph nodes (2). This entity is characterized histologically by large reactive follicles with mantle zone small B-cells and remnants of large germinal center cells infiltrating into germinal centers (2). Its mantle zone is obscured. Its interfollicular areas usually contain small lymphocytes and a few immunoblasts (12).
PTGC commonly presents in adolescent and young adult males as solitary painless lymphadenopathy of various durations. It is usually found in lymph nodes of patients with reactive follicular hyperplasia (23). Cervical nodes are the most commonly involved ones while involvements of axillary and inguinal nodes are less frequent (2). To the best of our knowledge, PTGC in the presacral space has not been reported previously in the literature. Here, we report a rare case of PTGC presenting as a soft tissue mass in the presacral space.
A 37-year-old female patient was admitted to our hospital with a chief complaint of lower abdominal pain since one day prior to the hospital visit. She had no relevant medical or surgical history except cesarean section 10 years before. She was not on medications. She had no family history of malignancy. She had a normal heart rate at 76 beats per minute with blood pressure of 120/84 mmHg. She was normopnoeic at 20 breaths per minute and afebrile. There was direct tenderness in the lower abdomen without rebound tenderness.
Acute gastroenteritis was suspected clinically. Contrast enhanced abdomen and pelvis computed tomography (CT) was performed. CT images demonstrated out-pouching and enhancing lesions at the proximal ascending colon with severe omentomesenteric infiltrations and peritoneal thickenings. Acute diverticulitis was suspected. A well-circumscribed and mildly homogeneously enhanced soft tissue mass measuring at about 3.5 cm was incidentally detected in the presacral space on CT. No central necrosis or calcification was identified in the mass.
The patient became asymptomatic after receiving antibiotics as a treatment for acute diverticulitis. During hospital admission, pelvis magnetic resonance imaging (MRI) was performed using 3T system (Achieva, Philips Healthcare, Best, the Netherlands) to evaluate the mass lesion in the presacral space. MRI demonstrated a soft tissue mass with slightly high signal intensity on T2-weighted images and iso-signal intensity on T1-weighted images. Gadolinium-enhanced T1-weighted imaging showed mild and inhomogeneous enhancement in 1-min delayed phase. Diffusion-weighted image (DWI) showed high signal intensity with a high b-value (800 s/mm2) but a low signal intensity on apparent diffusion coefficient (ADC) map. In addition, there were a few small foci with high T2 signal intensity showing strong contrast enhancement. They were scattered in the center of the mass. These foci were not shown on DWI or ADC map because of their small sizes (Fig. 1).
Taken together, imaging features of a mass in the presacral space on CT and MRI initially suggested a neurogenic tumor such as schwannoma or Castleman's disease. A robotic excisional biopsy was performed and result was uneventful. The mass was located in the presacral space with feeding vessels from the superior rectal artery. It was completely excised with clear and negative margins.
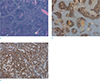
On gross examination, the specimen consisted of an enlarged lymph node measuring at 3.6 × 3.3 × 1.8 cm. On sectioning, the cut surface showed a diffuse yellowish-gray homogeneous creamy appearance (Fig. 2). On microscopic examination, the enlarged lymph node showed a florid follicular hyperplasia pattern. In central areas, several thick-walled blood vessels with occasional hyalinization were observed. The lymph node showed variable sizes and enlarged follicles with indistinct margins that were highlighted by CD21 immunostaining. A small amount of transformed germinal centers had secondary germinal centers. Some had a coalescent appearance. A considerable amount of small-sized lymphocytes extended into the periphery of the enlarged germinal centers. To rule out the possibility of follicular lymphoma, bcl-2 immunostaining was performed. Cells of reactive lymphoid follicles in the germinal center were not positively stained. However, bcl-2 positive mantle zone B cells were observed in the blurred periphery of germinal centers. Based on these findings, a diagnosis of PTGC was made (Fig. 3).
PTGC is an entity presenting with a well-defined histologic pattern of follicular changes often associated with reactive lymphadenopathy. In PTGC, a single lymph node is typically affected, although multiple lymph nodal involvement has been reported (3). The most common site involved is in the cervical region. The axilla and inguinal regions, skin, and oral cavity are less commonly involved. Abdominal lymph nodes are infrequently affected (12). No case of PTGC in the presacral space or pelvic cavity have been previously reported in the literature.
Image findings of PTGC are not always documented. In previous studies (456), CT has been used to demonstrate PTGC lesions as homogeneous enhancing soft tissue masses. MRI has revealed that they have iso-signal intensity on T1-weighted images with slightly high signal intensity on T2-weighted images. Image findings of the present case are similar to those reported by Park et al. (4). PTGC in our case also showed mild contrast enhancement on contrast-enhanced T1-weighted images and diffusion restriction on diffusion-weighted images, similar to those in the findings of Park et al. (4).
The presacral space is clinically important. It consists of the axial skeleton, neural axis, and pelvic soft tissues. This space contains osteochondral tissues from the sacrum and coccyx, neural tissues from the cauda equina and branches of the sacral plexus, and mesenchymal tissues from adjacent organs. It also contains surrounding connective tissues and blood and lymphatic vessels (7). A wide variety of benign and malignant conditions may occur from these various elements (7). In the present case, there was no aggressive feature such as adjacent organ invasion or bony destruction around the mass. Therefore, we considered benign conditions in the differential diagnosis, including neurogenic tumor and Castleman's disease. Castleman's disease is a disorder of nonclonal lymph node hyperplasia that primarily involves lymphatics, although extralymphatic sites such as lungs, larynx, parotid glands, pancreas, meninges, and muscles might be involved (789). It commonly appears as a single enlarged lymph node or conglomerate nodal mass with intense and homogeneous contrast enhancement. Internal calcifications may be seen in as many as 10% of lesions (789). Castleman's disease and neurogenic tumors usually show strong and homogeneous contrast enhancement. However, PTGC in our case demonstrated a mild and inhomogeneous contrast enhancement. Focally and strongly enhancing foci in the center of the PTGC corresponded to an area with several thick-walled blood vessels with occasional hyalinization on pathologic examination.
It has been reported that PTGC has significant association with lymphoma. Nodular lymphocyte-predominant Hodgkin lymphoma has been diagnosed in 35% of adults with PTGC, although there remains no evidence supporting a common pathogenesis between PTGC and lymphoma (2). Both conditions can present in a similar way. They should be differentiated on the basis of radiologic, histologic, and immunologic analysis. Follow-up and repeat biopsy are needed for such cases to confirm the recurrence of PTGC or lymphocyte predominant Hodgkin's lymphoma (3).
In conclusion, we report a unique case of PTGC in the presacral space presenting as a well-defined mass with slightly high signal intensity on T2-weighted images and iso-signal intensity on T1-weighted images. It showed mild contrast enhancement and diffusion restriction on DWI. Awareness of these imaging features of PTGC may help radiologists make differential diagnosis of a solitary mass lesion in the presacral space.
Figures and Tables
Fig. 1
Axial T2-weighted turbo spin-echo (a) and axial T1-weighted turbo spin-echo (b) MR images showing a well-circumscribed presacral mass with slightly high signal intensity on T2-weighted imaging and iso-signal intensity on T1-weighted imaging. Axial contrast-enhanced T1-weighted subtraction MRI (c) showing homogeneous enhancement on 1-min delayed phase. DWI (b-value = 800 s/mm2) (d) showing high signal intensity in the mass. (e) The mass demonstrates low signal intensity on ADC map. A few small high T2 signal intensity foci (a, arrow) showing strong contrast enhancement (c, arrow) were scattered in the center of the mass. These foci were not shown on DWI or ADC map due to their small sizes.
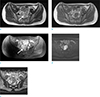
Fig. 2
The specimen with a round shaped soft tissue measured at 3.6 × 3.3 × 1.8 cm. The external surface is smooth. On section, the cut surface shows a diffusely yellowish-gray homogeneous appearance.

Fig. 3
The section from lymph nodes with variable sizes and enlarged follicles with indistinct margins. It is composed of follicular mantle lymphocytes and extensive follicular dendritic cells. H&E stain, × 40 (a). Positive CD21 immunostaining of follicular dendritic cells, × 40 (b). Bcl-2-positive mantle zone B cells infiltrate and disrupt germinal centers, × 200 (c).
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). No benefit in any form has been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. The authors declare that they have no competing interests. Please address correspondence and reprint requests to Deuk Jae Sung.
References
1. Lennert K, Muller-Hermelink HK. Lymphocytes and their functional forms - morphology, organization and immunologic significance. Verh Anat Ges. 1975; 69:19–62.
2. Leong A. A pattern approach to lymph node diagnosis. New York: Springer;2011.
3. Hicks J, Flaitz C. Progressive transformation of germinal centers: review of histopathologic and clinical features. Int J Pediatr Otorhinolaryngol. 2002; 65:195–202.
4. Park SW, Jang SM, Kim DY, Son JH, Cho YC, Sung IY. Progressive transformation of germinal centers in submandibular area. J Korean Assoc Maxillofac Plast Reconstr Surg. 2011; 33:368–372.
5. Miller MW, Gatter KM, Cannady SB, Wax MK. Progressive transformation of germinal centers (PTGC) in the head and neck. Laryngoscope. 2010; 120:Suppl 4. S168.
6. Chang CA, Kumar B, Nandurkar D. A case report of high 18F-FDG PET/CT uptake in progressive transformation of the germinal centers. Medicine (Baltimore). 2015; 94:e412.
7. Hain KS, Pickhardt PJ, Lubner MG, Menias CO, Bhalla S. Presacral masses: multimodality imaging of a multidisciplinary space. Radiographics. 2013; 33:1145–1167.
8. Bonekamp D, Horton KM, Hruban RH, Fishman EK. Castleman disease: the great mimic. Radiographics. 2011; 31:1793–1807.
9. Pickhardt PJ, Bhalla S. Unusual nonneoplastic peritoneal and subperitoneal conditions: CT findings. Radiographics. 2005; 25:719–730.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download