Abstract
Chronic expanding organizing hematoma (CEH) occasionally mimics a soft tissue tumor on MRI, which becomes more problematic in patients with a history of surgical resection for musculoskeletal malignancy. Herein, we present a case of CEH which we were able to differentiate from recurrent tumor through MRI follow-up, including diffusion-weighted imaging (DWI) and dynamic contrast enhanced (DCE) imaging. A 66-year-old male visited our institution under suspicion of recurrent leiomyosarcoma of the thigh, 19 months after surgery and radiation therapy. Due to inconclusive results, three US-guided biopsies and 6 MRI examinations were performed over 2 years. In the end, we could diagnose a CEH using conventional and functional MRI techniques, and it was histopathologically confirmed after surgical resection. A CEH may occur remotely after an initiating event, and it may persist and expand over several years. Functional MR sequences, in addition to conventional sequences, are helpful in differentiating CEH from malignant neoplasms.
Chronic expanding organizing hematoma (CEH), also known as chronic expanding hematoma, is a benign condition in which a hematoma does not undergo resorption, but rather it persists or even expands slowly. The term was first coined by Reid et al. (1), and the authors defined CEHs as hematomas that increase in size for over 1 month after the causative event. The clinical importance of this lesion arises from the fact that it often mimics malignant soft tissue neoplasms, both clinically and radiologically (234).
Numerous studies have described the features of CEH on conventional MRI (234), but reports that present the findings on diffusion-weighted imaging (DWI) (3) and dynamic contrast-enhanced (DCE) imaging are rare. Herein, we illustrate an unusual case of CEH in which we could obtain regular, serial MRI scans, including DWI and DCE, for over 2 years. This case demonstrates the added value of DWI and DCE for differentiating CEH from local recurrence of a malignant soft tissue neoplasm.
The key events and imaging findings are summarized in Figure 1.
A 66-year-old man initially visited a secondary hospital with a palpable mass in his right thigh, which was first noticed several months before. There was no history of trauma, and no medical or social history of interest. Conventional radiography showed a soft-tissue density bulge in the right medial thigh, without any specific imaging finding.
Contrast enhanced MRI was performed for further evaluation. MRI revealed a 5.5 × 5.4 × 3.7 cm-sized, relatively well-circumscribed mass with lobulated contours at the mid-thigh level, involving primarily the anterior compartment and also the medial compartment. The mass was homogeneous and slightly hyperintense to skeletal muscle on T1-weighted images, and heterogeneously hyperintense to skeletal muscle on T2-weighted images. A small area of cystic degeneration was also seen. After contrast administration, there was avid enhancement with mild heterogeneity. Contrast-enhanced T1-weighted images also revealed infiltrative peritumoral enhancement. There was no evidence of metastasis on positron emission tomography-computed tomography (PET-CT) (Fig. 2).
Under suspicion of a malignant soft tissue tumor, wide excision of the mass was performed in the same hospital. Leiomyosarcoma, pleomorphic type, was diagnosed after pathological examination. Following surgery, the patient received adjuvant radiation therapy.
Follow-up MRI was performed at 1, 4, 5, and 8 months after surgery, and until this period, the clinical course was uneventful.
Eighteen months after surgery, follow-up MRI and PET-CT were performed again. This time, three ill-defined, nodular enhancing lesions appeared in the surgical bed. The lesions measured 1-2.1 cm in diameter. These lesions were isointense to skeletal muscle on T1-weighted images and heterogeneous on T2-weighted images, and showed hypermetabolic activity on PET-CT. The possibility of recurrent malignancy was raised, and the patient visited our institute for further management.
After visiting the orthopedic outpatient clinic, imaging studies from the previous hospital were reviewed. The history, physical examination, and laboratory findings were non-specific. Ultrasound-guided percutaneous biopsy was requested for the suspected recurrent tumor. On ultrasound examination, the largest enhancing lesion detected on MRI was seen as an ill-defined hypoechoic lesion, rather than a mass-like lesion. However still, recurrent tumor could not be excluded and ultrasound (US)-guided biopsy was performed. Several days later, histopathologic examination confirmed that the lesion was benign tissue with fibrosis, and a clinicoradiologic follow-up was planned.
The first follow-up MRI at our hospital was obtained 3 months after US-guided biopsy. The previously observed focal enhancing lesions in the operation bed were persistently noted, among which 2 lesions did not show a significant change in MR signal characteristics and size. However, the largest lesion showed a more discrete margin on enhanced T1-weighted images. Since the overall extent had not changed despite any further treatment, we decided to perform imaging follow-up once more.
MRI follow-up was performed after 3 months and 2 weeks. Again, the extent and characteristics were not remarkably different compared to the MRI examination performed at the first follow-up in our hospital. But compared to the follow-up images taken 18 months after surgery at the previous outside hospital, a slight increase in size was suspected. This time, quantitative DWI analysis was also performed for the largest lesion, which showed a relatively high mean apparent diffusion coefficient (ADC) value of 1.67 × 10-3 mm2/sec. PET-CT obtained 2 weeks prior to MRI showed mild hypermetabolic activity, not in, but adjacent to, the largest lesion (Fig. 3). We presumed that these lesions were some sort of a tumorous condition with myxoid degeneration, and re-biopsy was planned.
Second US-guided biopsy was performed after an interval of 2 weeks. As noted on MRI, the largest lesion showed a relatively well-circumscribed appearance. Biopsy was successful without any complications. The subsequent pathology report was again nonspecific, with chronic inflammation with fibrosis as the impression.
Follow-up MRI was obtained after 3 months. The largest lesion showed an increase in size, and urgent surgical intervention was planned. But PET-CT follow-up performed after 1 week reported a decrease in SUVmax, which was discordant with the MRI results. An interdepartmental conference was held, and the possibility of a CEH was considered over the possibility of tumorous pathology. Under this impression, US-guided biopsy was planned once more, rather than surgery.
The third US-guided biopsy was successfully performed 1 month after the last MRI (1 day after the conference); the pathology report was the same as before, reporting chronic inflammation with fibrosis.
Under the diagnosis of a probable benign lesion, regular MRI follow-up was planned. On the fourth follow-up (34 months post-operatively), no significant change in lesion characteristics was noted.
However, on the fifth follow-up (37 months and 2 weeks post-operatively), size of the largest lesion had increased. Also, a newly developed smaller mass, with same imaging features as the largest lesion, was observed at a more proximal location between the adductor longus and the vastus medialis. We decided that although previous biopsy results were benign, all these lesions could be recurrent tumors, as it is unusual for organizing hematomas to increase in number, so remotely after the causative event. Surgery was planned after 2 weeks, but ultimately the operation was postponed due to a severe respiratory infection.
Four months and one week later, MRI was performed for pre-operative evaluation. The largest lesion in the right adductor muscle had again increased in size. However, size of the recently developed lesion between the adductor longus and the vastus medialis had decreased. On T2-weighted images, the largest lesion showed a dark signal intensity rim, which was not acknowledged on previous MRI scans. On DWI, the mean ADC value was 1.9 × 10-3 mm2/sec. DCE imaging revealed nonarterial enhancement (Fig. 4). These findings were interpreted as a sign of low cellularity and vascularity not associated with malignancy. The final radiological impression was CEH, and surgery was performed after 1 week. The operation record noted a brownish mass between the adductor muscles.
Microscopically, the lesion was surrounded by a thick fibrous capsule with chronic inflammation and granulation tissue. Fibrinous exudates with hemorrhage, dilated vessels, and neovascularization were identified within the lesion. On the basis of these findings, the diagnosis of CEH could be confirmed (Fig. 5).
CEHs can occur due to a variety of causes, including bleeding diathesis, anti-coagulation, trauma, surgery, radiation therapy, or they may develop spontaneously (25). Possible mechanisms behind their persistence and growth have been described; however, it is still unclear (234). Under the microscope, these lesions consist of a dense fibrous capsule and internal contents, which in turn is an amalgam mainly composed of blood breakdown products, connective tissue, granulation tissue, and necrotic debris (234). These histologic characteristics are reflected in the MRI features. The central part of the lesion shows a heterogeneous signal on both T1- and T2-weighted images. The peripheral capsule is frequently seen as a thick rim with dark signal intensity on T2-weighted images, due to hemosiderin deposits and iron-laden macrophages. On contrast-enhanced studies, these masses often show peripheral enhancement, and in some cases, they show internal enhancement (3).
Apparently, CEH can mimic a soft tissue neoplasm and it can frequently impede the diagnostic process. This becomes more confusing in cases in which the lesions occur in the surgical bed of a previously known malignancy. CEH versus local recurrence becomes a critical issue, in terms of deciding further treatment options and aggressiveness of the salvage operation.
Features on conventional radiography, angiography, CT, US, and MRI do not provide sufficient diagnostic specificity (234). Although some MRI features, such as the dark signal intensity rim on T2-weighted images, favor the diagnosis of CEH, this feature is not always present (3). Findings on fluorodeoxyglucose (FDG)-PET are not well established, but one case report showed that a significantly increased FDG uptake can be seen at the periphery, mimicking a malignant soft tissue tumor (6). Core biopsy is a minimally invasive method for histopathological diagnosis of soft tissue masses, but it has an inherent sampling error. Rapidly growing sarcomas often undergo partial necrosis due to insufficient angiogenesis, and sampling of these areas results in non-diagnostic specimens (7). Moreover, sarcomas are difficult to diagnose on the basis of cellular morphology alone (7). Most cases of CEH reported in the literature were diagnosed by incisional biopsy or surgical excision (234), with diagnosis suggested by percutaneous biopsy being an infrequent event.
Multiple recent publications have demonstrated the incremental value of DWI for distinguishing malignant soft tissue tumors from benign soft tissue tumors. Quantitative evaluation using the ADC value is the key method, although the cut-off value and diagnostic performance vary among studies (38). To date, only one study has specifically focused on DWI of CEH, and it reported a mean value of 1.55 × 10-3 mm2/sec for CEH, which was significantly different from that of malignant soft tissue tumors (0.92 × 10-3 mm2/sec) (3).
DCE imaging is another functional MR sequence of recent interest. Qualitatively, presence of arterial enhancement on maximum intensity projection images and raw acquired time phases are evaluated (9). Quantitative analysis includes measurement of various parameters derived from DCE, such as the volume transfer constant between the blood plasma and the extracellular/extravascular space (Ktrans), and inspection of the time-concentration curve plots (8). Its value for differentiating malignant soft tissue tumors from benign soft tissue tumors has been validated, but DCE features of CEH have not yet been published. A study by Del Grande, et al. (9) reported arterial enhancement in 2 out of 12 cases of postoperative hematoma, and nonarterial enhancement in 10 out of 12 cases of postoperative hematoma.
Our case is a unique addition to the existing literature on CEH due to two key points. First, a relatively regular MRI follow-up was performed over 2 years, without any therapeutic intervention. This enabled us to inspect the temporal evolution of CEH on MRI. We can cautiously presume that before organization of CEH, the lesion starts out as an ill-defined focal area of abnormal signal intensity and enhancement. Over time, the lesion develops a pronounced mass-like appearance, and later on, it shows a dark signal intensity rim representing the hemosiderin-laden capsule. In view of the smaller lesion that appeared during the fifth follow-up, we can surmise that CEH may occur remotely after the triggering event. Although associating the new lesion with surgery and radiation therapy performed 3 years prior may seem implausible, no other traumatic event except for percutaneous biopsy was documented during the follow-up period, and the patient did not have any underlying coagulopathy. The new lesion was separate from the biopsied mass and it was well clear of the biopsy tract; therefore, the possibility of percutaneous biopsy being the traumatic event is less likely. Reports on radiation-induced CEHs characteristically document a relatively long interval between treatment and CEH development, which supports the possibility of radiation therapy being the most likely cause (5). Decreased size on the sixth follow-up demonstrates the possibility that spontaneous resorption may also occur.
The second point of interest in this case is the value of DWI and DCE. Before the development of the characteristic dark signal intensity rim, which was a late event, high mean ADC values were persistently noted from the first follow-up. High ADC values are usually seen in benign lesions, with some exceptional cases such as those of myxoid liposarcoma. The primary malignancy in our case was leiomyosarcoma, which is not included in these exceptions (10).
DCE was performed during all MRI examinations at our institute, but quantitative analysis was performed only for the last follow-up. In agreement with previous studies of DCE, our case showed a slow, gradual enhancement on the dynamic enhancement curve (8). These features may also have been present early in the follow-up, and they could have helped in suggesting the benignity of the lesion.
In the end, decrease in lesion size and development of a dark signal intensity rim were the essential findings for making a confident diagnosis of benignity. But along the clinical course, functional MR sequences provided valuable information that weighed against recurrent tumor. We believe that DWI and DCE can be helpful in equivocal cases, and they may aid in deciding the best course of action for the clinician and the patient.
We conclude that functional MR sequences are helpful in the early differential diagnosis of CEH from local recurrence of soft tissue malignancy. DWI and DCE, along with conventional MR sequences, will help establish the proper diagnosis and treatment plan for patients with CEH.
Figures and Tables
 | Fig. 1Overview of the patient's clinical course. CEH = chronic expanding organizing hematoma; Chr. infl. = chronic inflammation; Inc size = increased size; M = month; US-Bx = US-guided biopsy; W = week |
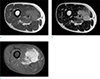 | Fig. 2Initial MRI obtained at the previous hospital. T1-weighted image (a), T2-weighted image (b), and T1-weighted contrast-enhanced image (c). There is a well-circumscribed mass with lobulated contours in the mid-thigh. The pathologic diagnosis was leiomyosarcoma. |
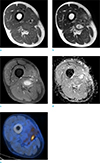 | Fig. 3Second MRI obtained at our institute. T1-weighted image (a), T2-weighted image (b), T1-weighted contrast-enhanced image (c), apparent diffusion coefficient (ADC) map (d), and PET-CT obtained two weeks before (e). An enhancing nodular lesion in the operative bed is seen. This lesion shows an increase in size, compared to the previous MRI examination (not shown). The ADC map does not show diffusion restriction. |
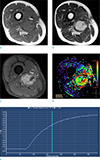 | Fig. 4Sixth (last) MRI obtained at our institute. T1-weighted image (a), T2-weighted image (b), T1-weighted contrast-enhanced image (c), apparent diffusion coefficient (ADC) map (d), and dynamic enhancement curve (e). The growing mass now shows a distinct hypointense rim on T2-weighted images, which is a characteristic finding of chronic expanding organizing hematoma. The high ADC value and non-arterial enhancement curve favor benignity. |
 | Fig. 5Low-power microscopic photograph of the surgical specimen. There is chronic inflammation and granulation tissue, with a surrounding thick fibrous capsule. Fibrinous exudates with hemorrhage, dilated vessels, and neovascularization were seen within the lesion. Pathology report confirmed the diagnosis of chronic expanding organizing hematoma. |
References
1. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980; 244:2441–2442.
2. Sreenivas M, Nihal A, Ettles DF. Chronic haematoma or soft-tissue neoplasm? A diagnostic dilemma. Arch Orthop Trauma Surg. 2004; 124:495–497.
3. Oka K, Yakushiji T, Sato H, et al. Ability of diffusion-weighted imaging for the differential diagnosis between chronic expanding hematomas and malignant soft tissue tumors. J Magn Reson Imaging. 2008; 28:1195–1200.
4. Aoki T, Nakata H, Watanabe H, et al. The radiological findings in chronic expanding hematoma. Skeletal Radiol. 1999; 28:396–401.
5. Park JC, Ahn JS, Kwon DH, Kwun BD. Growing organized hematomas following gamma knife radiosurgery for cerebral arteriovenous malformation: five cases of surgical excision. J Korean Neurosurg Soc. 2015; 58:83–88.
6. Nishida Y, Kobayashi E, Kubota D, et al. Chronic expanding hematoma with a significantly high fluorodeoxyglucose uptake on 18F-fluorodeoxyglucose positron emission tomography, mimicking a malignant soft tissue tumor: a case report. J Med Case Rep. 2014; 8:349.
7. Kasraeian S, Allison DC, Ahlmann ER, Fedenko AN, Menendez LR. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res. 2010; 468:2992–3002.
8. Kim JI, Lee IS, Song YS, Park SK, Choi KU, Song JW. Short-term follow-up MRI after unplanned resection of malignant soft-tissue tumours; quantitative measurements on dynamic contrast enhanced and diffusion-weighted MR images. Br J Radiol. 2016; 89:20160302.
9. Del Grande F, Subhawong T, Weber K, Aro M, Mugera C, Fayad LM. Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology. 2014; 271:499–511.
10. Surov A, Nagata S, Razek AA, Tirumani SH, Wienke A, Kahn T. Comparison of ADC values in different malignancies of the skeletal musculature: a multicentric analysis. Skeletal Radiol. 2015; 44:995–1000.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download