Abstract
Periductal stromal sarcoma (PSS) is a type of rare malignant fibroepithelial tumor. PSS is a recently introduced diagnostic entity and there are few reports about radiological features of this tumor. Pre-operative diagnosis is difficult because it reveals similar symptoms with other benign and malignant tumors with absence of specific radiologic findings. We present a woman age 30 that underwent mammotome biopsy for a BI-RADS 4 lesion on her left breast and received histopathology diagnosis of a phyllodes tumor. Additionally, she underwent a wide excision depending on her histopathology diagnosis. Her final diagnosis was PSS. Six months later, no recurrence was detected. However, frequent follow-up is needed because PSS can develop into phyllodes tumor or entity of breast cancer.
Periductal stromal sarcoma (PSS) is an extremely rare, low grade neoplasm that arises from breast connective tissue, specifically from the periductal stroma. PSS has a biphasic morphology with benign ducts and a sarcomatous stroma and was classified as a different entity by the World Health Organization (WHO) in 2002 because of lack of phyllodes architecture. Absence of specific radiologic findings make the diagnosis difficult (1234). We reviewed a few case reports and present radiologic features.
A 30-year-old woman presented to outside breast clinic with a palpable lump in the left breast. She had been diagnosed with fibromatous hyperplasia via gun biopsy at the outside breast clinic 1 week ago. Patient was referred to our hospital for further evaluation. On physical examination, the tumor was in the left breast 1 o'clock direction, well circumscribed, mobile and non-tender. Outside mammogram was of limited evaluation due to extremely dense breasts (Fig. 1a). There was no associated calcifications or axillary-lymphadenopathy. On breast sonography conducted in our hospital, there was a 22 × 10 mm-sized oval, hypoechoic mass with parallel orientation and angular margin in the 1 o'clock direction of the left breast, 2 cm from the nipple (Fig. 1b). The lesion was classified as a category 4 per the American College of Radiology Breast Imaging-Reporting and Data System (ACR BI-RADS) criteria. A mammotome biopsy was conducted for the palpable hypoechoic lesion in the left breast, and the lesion was confirmed as phyllodes tumor with marked stromal atypia. And breast magnetic resonance imaging (MRI) was conducted using a 3.0-T scanner with patient maintained in the prone position after mammotome excision. Both irregular post-mammotome change and a 6mm-sized irregular shaped mass, revealing high signal intensity on fat-saturated T2-weighted image (T2WI) (Fig. 2a) and iso-signal intensity on the T1-weighted image (T1WI), were revealed in upper outer portion of the left breast. Additionally, the mass revealed homogeneous enhancement (Fig. 2b) with wash-out pattern on kinetic curve assessment, and diffusion restriction on diffusion-weighted image. There were no significantly enlarged lymph nodes in either axillae. We assessed the lesion as remained tumor. Possibility of malignancy was suggested and complete excision was recommended. Therefore, wide excision was conducted.
Microscopic examination revealed the tumor had biphasic proliferation and a multinodular growth pattern composed of spindle cells without significant atypia, cuff formations around open tubules without leaf-like growth pattern and mitotic activity up to 3/10 high power fields (HPF) (Fig. 3a, b). Epithelial components had a double layer or hyperplastic features without atypia and leaf-like growth pattern. On immunohistochemistry staining, ductal cells were positive for CD34 and negative for estrogen receptor (ER), progesterone receptor (PR) and S100 (Fig. 3c, d). Proliferation index (Ki-67) was 5–6%. According to histologic criteria that was defined by the Armed Forces Institute of Pathology (AFIP), the diagnosis of PSS was established.
PSS was previously accepted as a synonym for phyllodes tumor, but it was recently classified as a distinct entity by the WHO in 2002 because of lack of leaf-like growth pattern (12). Unlike phyllodes tumor, incidence of PSS is higher in peri-menopausal and post-menopausal women. Conversely, it is like phyllodes tumors in that it can develop into sarcoma, and that it is prone to local recurrence if imperfectly removed. Common symptoms of PSS are like other benign and malignant breast tumors (2). There were no specific radiologic findings for PSS because PSS is an extremely rare entity and only a few cases were reported.
In our case, PSS presented as an oval and hypoechoic mass with parallel orientation and angular margin on ultrasonography. There were limitations in evaluating mammogram and MRI because of extremely dense breasts and surgical excision before scanning MRI. However, on MRI, remnant PSS revealed high signal intensity on T2WI and washout enhancement pattern on kinetic study. A few case reports described that radiologic features of PSS resembled with fibroadenoma.
Histologically, it has a biphasic proliferation with spindle cells around open ducts, tubules, and cellular sarcomatous stroma (56). Histologic criteria for PSS according to the AFIP include: predominantly sarcomatous spindle cell stromal proliferation around open ducts and tubules, lack of leaf-like growth pattern, one or more nodules that can be separated by adipose tissue, mitotic activity equal to or more than 3 in 10 HPF and infiltration into surrounding adipose tissue (23). Histologic grading depends on atypia and mitotic count ranges from low to high grade. Tumor cells are only positive with CD34, CD10, and lacks CD117, S-100, ER, and PR expression (27).
PSS is a tumor of intermediate behavior, resected with considerable margin. But, axillary lymph node dissection is usually not required. Adjuvant chemotherapy or radiotherapy is not recommended (23). The tendency of PSS to recur and progress into phyllodes tumor of soft tissue sarcomas, as well as the occasional appearance of intraepithelial changes ranging from ordinary hyperplasia to intraductal carcinoma (238), indicate that close follow-up is required. In our case, patient was treated with surgical resection at our hospital. Resection was conducted with sufficient margins and patient has been recurrence-free for approximately 6 months.
Differential diagnosis of PSS includes phyllodes tumor, spindle cell carcinoma, myoepithelial carcinoma and other breast sarcomas (1). The main difference from phyllodes tumor is lack of leaf-like pattern. Differential diagnosis from spindle cell carcinoma is crucial due to different targeted therapy. Immunohistochemistry can be beneficial for diagnosis; tumor cells are positive with CK5, CK14, p63, and negative with CD34 in spindle cell carcinoma (910). Breast sarcomas have highly pleomorphic nuclei, abundant mitotic figures, positive with CD10 and Vimentin, negative with CD34. Myoepithelial carcinomas have infiltrative growth pattern with biphasic or glandular pattern and are highly pleomorphic, mitotically active, positive for Vimentin, SMA, S100, p63 and CK14 (110). In our case, tumor composed of minimal atypical spindle cells surrounded open ducts and tubules without leaf-like growth pattern. Spindle cells were only positive with CD34, negative with ER, PR and S100, and proliferation index was 5–6%, compatible with PSS.
In conclusion, PSS is recently introduced diagnostic entity and there are few reports about radiological features of this tumor. Pre-operative diagnosis is difficult because it reveals similar symptoms with other benign and malignant tumors with absence of specific radiologic findings. Extensive tumor sampling and additional immunohistochemistry should be conducted for appropriate diagnosis.
Until recently, surgical resection with a significant margin has been the best treatment so far because treatment strategy of PSS has not been established correctly. Therefore, it is expected that more research and case reports will be needed including close observation on PSS.
Figures and Tables
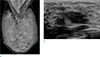 | Fig. 1(a) Mammogram mediolateral oblique (MLO) view reveals extremely dense breasts that make it difficult to evaluate the mass lesion. (b) Ultrasonographic image reveals oval mass in parallel orientation with angular margins in upper outer quadrant of the left breast. |
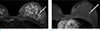 | Fig. 2On MRI of breasts after mammotome excision, a 6 mm-sized irrgular shaped mass (arrow), revealing high signal intensity on fat-saturated T2-weighted image (a) with homogeneous enhancement (arrow) on first dynamic enhancement study (90 seconds) (b) was found in upper outer quadrant of the left breast. |
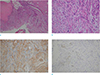 | Fig. 3On pathology examination, (a) low-power field (× 40) and (b) high-power field (× 200) images of the tumor reveal biphasic proliferation and a multinodular growth pattern and was composed of spindle cells without significant atypia, cuff formations around open tubules without leaf-like pattern. (c) On immunohistochemistry staining, stromal cells were positive with CD34 and (d) negative with S100. |
References
1. Tavassoli FA, Devilee P. Pathology and genetics tumours of the breast and female genital organs. In : Tavassoli FA, Devilee P, editors. World Health Organization classification of tumours. Lyon: IARC Press;2003. p. 101–102.
2. Burga AM, Tavassoli FA. Periductal stromal tumor: a rare lesion with low-grade sarcomatous behavior. Am J Surg Pathol. 2003; 27:343–348.
3. Rao AC, Geetha V, Khurana A. Periductal stromal sarcoma of breast with lipoblast-like cells: a case report with review of literature. Indian J Pathol Microbiol. 2008; 51:252–254.

4. Pandey M, Mathew A, Abraham EK, Rajan B. Primary sarcoma of the breast. J Surg Oncol. 2004; 87:121–125.

5. Callery CD, Rosen PP, Kinne DW. Sarcoma of the breast. A study of 32 patients with reappraisal of classification and therapy. Ann Surg. 1985; 201:527–553.
6. Oberman HA, Nosanchuk JS, Finger JE. Periductal stromal tumors of breast with adipose metaplasia. Arch Surg. 1969; 98:384–387.

7. Tomas D, Jankovic D, Marusic Z, Franceschi A, Mijic A, Kruslin B. Low-grade periductal stromal sarcoma of the breast with myxoid features: Immunohistochemistry. Pathol Int. 2009; 59:588–591.

8. Masbah O, Lalya I, Mellas N, et al. Periductal stromal sarcoma in a child: a case report. J Med Case Rep. 2011; 5:249.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download