Abstract
Idiopathic brain herniation is a rare condition. We believe that this is the first reported case of idiopathic herniation of the lingual gyrus. The case involves a 57-year-old woman presenting with frontal headache without overt visual symptoms. Magnetic resonance imaging (MRI) revealed an idiopathic herniation of the lingual gyrus of the occipital lobe extending into the quadrigeminal cistern. No other adjacent intracranial abnormalities were observed. Although some conditions may be considered in the differential diagnosis, accurate diagnosis of idiopathic brain herniation in medical practice can prevent unnecessary additional imaging procedures and invasive open biopsy in patients with typical imaging findings.
Idiopathic brain herniation is rare. Its exact incidence is unknown. Maldjian et al. (1) recently reported a prevalence rate of 0.73% (11/1500) for idiopathic herniation of the cuneate gyrus of the occipital lobe, based on computed tomography (CT) images.
Idiopathic gyral herniation can be detected using characteristic imaging findings using CT or magnetic resonance imaging (MRI). These findings include a focal bulging area of soft tissue attenuation or signal intensity that is continuous with the adjacent brain parenchyma extending into the cisternal space (23). In addition, the herniated gyral segment shows similar attenuation or signal intensity as the brain parenchyma with or without a dural defect (23).
We describe a case of a unilateral idiopathic herniation of the lingual gyrus of the occipital lobe through the quadrigeminal cistern using MRI. To our knowledge, such a case has not been described before.
A 57-year-old, previously healthy woman presented to the neurology department with a 3 week history of frontal headache and nausea without any neurologic deficits. The patient did not have any past history of head trauma, surgery, or infection that may have caused the brain herniation. Routine blood test results were normal. The patient's body temperature was 36.5℃, blood pressure was 112/77 mmHg, and pulse rate was 81 beats/minute. She was alert and did not have neck stiffness. Her numerical rating scale score for pain was 2. Neurological examinations, including cranial nerve function tests, did not show any neurologic abnormalities. Electroencephalography and cerebrospinal fluid examination was not performed.
The patient was scanned using a 3.0-T MR scanner (Achieva; Philips Healthcare; Best, the Netherlands). The acquisition parameters for the axial T1-weighted turbo spine echo (TSE) sequence were as follows: repetition time (TR), 500.0 ms; echo time (TE), 10.6 ms; section thickness, 5.0 mm; spacing, 2.0 mm; matrix, 340 × 192; field of view (FOV), 220 × 220 mm; and number of excitations (NEX), 1. The acquisition parameters for the axial T2-weighted TSE sequence were as follows: TR, 3000.0 ms; TE, 80.0 ms; section thickness, 5.0 mm; spacing, 2.0 mm; matrix, 512 × 324; FOV, 220 × 220 mm; and NEX, 1. A three-dimensional (3D) T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence with contrast administration (Dotarem [gadoterate meglumine]) was acquired for additional axial and coronal multiplanar reconstruction (MPR). This sequence had the following parameters: TR, 9.9 ms; TE, 4.6 ms; section thickness, 1.0 mm; spacing, 0 mm; matrix, 240 × 240; FOV, 240 × 240 mm; and NEX, 1. A focal bulging contoured soft tissue signal intensity lesion continuous with the lingual gyrus of the right occipital lobe protruding into the quadrigeminal cistern was found by axial and coronal 3D T1-weighted MPRAGE imaging. This finding indicated brain herniation (Fig. 1a, b). No signal changes or abnormally enhancing lesions were evident in the herniated occipital lobe or the adjacent brain parenchyma. The herniated lingual gyrus measured 5.39 × 6.71 × 8.63 mm. A definite dural defect was not identified on the axial or coronal T1-weighted MPRAGE images or the axial T2-weighted fluid-attenuated inversion recovery (FLAIR) images (Fig. 1a–c). The patient's symptoms improved without any specific medical therapy, as only analgesics were administered. She is currently undergoing outpatient observation.
Cerebral herniation, commonly referred to as acquired brain herniation, is a displacement of cerebral tissue from its normal location into an adjacent space as a result of high-volume pressure. This herniation can be caused by several factors and can manifest in the presence or absence of increased intracranial pressure caused by traumatic brain injury, brain swelling, tumor, or intracranial hemorrhage. In contrast to acquired brain herniation, idiopathic brain herniation is rare and is incidentally diagnosed in various clinical conditions (1). The exact prevalence of idiopathic brain herniation is unknown. Maldjian et al. (1) reported that 11 of 1500 patients (0.73%) had idiopathic cuneate gyrus herniation based on emergency room CT examinations. Clinical manifestations of acquired brain herniation can vary depending on the location and degree of the herniated brain parenchyma. Idiopathic brain herniation leads to nonspecific clinical symptoms that vary from patient-to-patient (12345). However, the factors affecting the symptoms of idiopathic brain herniation are unknown.
The lingual gyrus of the occipital lobe is a small structure that plays an important role in visual processing (6). The present patient had no history of overt visual symptoms or related neurological deficits. So, formal visual field tests were not done.
The exact cause of the idiopathic gyral herniation could not be clearly identified. Several previous studies have suggested that errors during meningeal embryogenesis are responsible for this anomaly (23). The meninges of the brain first appear in the 4th week of embryonic development, as the embryo is derived from the mesoderm. Early in development, the telencephalon and diencephalon are separated by a layer of meninges. During development, when the telencephalon replaces the diencephalon, the mediating meningeal layer degenerates and the thalamus becomes continuous with the floor of the cerebrum (7). As the head of the embryo changes from a vertical to an oblique orientation, the median part of the tentorium disappears and the tentorium ultimately consists of only its lateral portions (8). The force regulating dural regression may locally influence the dura at the tentorial reflection. The resulting dural defect might then lead to focal brain parenchyma herniation (2).
CT scans and MRI are useful imaging techniques for the diagnosis of idiopathic brain herniation. Of the two modalities, MRI is the method of choice to evaluate idiopathic herniation of the brain. Moreover, characteristic and classical MRI features can be used to diagnose idiopathic brain herniation. A typical MRI characteristic of idiopathic brain herniation is a focal area of soft tissue signal intensity that is confluent with the nearby normal brain parenchyma and which extends into the adjacent cisternal space (1). In some cases, a focal dural defect can be directly identified without a bony defect. The herniated gyrus is isointense with the adjacent gyri in both pre- and post-contrast studies (1).
Idiopathic brain herniation may occupy the spaces confined by the meninges. It is necessary to differentiate between idiopathic brain herniation and intracranial or extracranial mass lesions, including astrocytomas, meningiomas, and metastases. The observed symptoms depend on the size of the lesion and the involvement of the cranial nerve. Radiologic findings alone can rarely distinguish between pathologic and non-pathologic lesions. Thus, open biopsy is an option for patients who do not choose surveillance through repeated imaging (4). In such cases, signal intensity identical to that of the adjacent cerebral parenchyma, absence of signal abnormalities, and contrast enhancement patterns on MRI of the idiopathic brain herniation may help to discriminate herniations from other pathologic masses. This, in turn, would ensure against the use of unnecessary surgical intervention (3).
In conclusion, we report the first case of idiopathic lingual gyrus herniation. In patients with idiopathic brain herniation, MRI reveals classical characteristic findings, such as a focal area of soft tissue signal intensity confluent with the adjacent normal brain parenchyma extending into the cisternal space with or without a dural defect. Although some differential diagnoses may be considered, accurate diagnosis of idiopathic brain herniation in medical practice can prevent unnecessary additional imaging procedures or invasive open biopsy in patients with typical imaging findings.
Figures and Tables
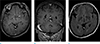 | Fig. 1Magnetic resonance images from a 57-year-old woman with idiopathic lingual gyrus herniation. The axial and coronal 3D T1-weighted MPRAGE images (a, b) with contrast administration show a focal bulging contour (indicated by arrows) continuous with the lingual gyrus of the right occipital lobe protruding into the quadrigeminal cistern. No definite dural defect was seen in the adjacent herniated gyrus (indicated by arrows) on axial or coronal 3D T1-weighted MPRAGE images or on axial T2-weighted FLAIR images (a–c). |
References
1. Maldjian C, Adam R. Prevalence of idiopathic cuneate gyrus herniation based on emergency room CT examinations. Emerg Radiol. 2014; 21:387–389.
2. Duarte MP, Maldjian TC, Tenner M, Adam R. Magnetic resonance imaging of idiopathic herniation of the cuneus gyrus. J Neuroimaging. 2007; 17:353–354.
3. Koc G, Doganay S, Bayram AK, et al. Idiopathic brain herniation. A report of two paediatric cases. Neuroradiol J. 2014; 27:586–589.
4. Horowitz M, Kassam A, Levy E, Lunsford LD. Misinterpretation of parahippocampal herniation for a posterior fossa tumor: imaging and intraoperative findings. J Neuroimaging. 2002; 12:78–79.
5. Yavarian Y, Bayat M, Brondum Frokjaer J. Herniation of uncus and parahippocampal gyrus: an accidental finding on magnetic resonance imaging of cerebrum. Acta Radiol Short Rep. 2015; 4:2047981614560077.
6. Blumenfeld H. Neuroanatomy through clinical cases. Disorders of higher-order visual processing. Sunderland, MA: Sinauer Associates, Inc.;2010. p. 914–917.
7. Larsen WJ. Human embryology. New York; Edinburgh: Churchill Livingstone;1997. p. 435–437.
8. O'Rahilly R, Muller F. The meninges in human development. J Neuropathol Exp Neurol. 1986; 45:588–608.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download