Abstract
Bone marrow aspirates concentrate (BMAC) transplantation is a well-known technique for cartilage regeneration with good clinical outcomes for symptoms in patients with osteoarthritis (OA). Magnetic resonance imaging (MRI) has an important role in evaluating the degree of cartilage repair in cartilage regeneration therapy instead of a second assessment via an arthroscopy. We experienced a case of hypertrophic regeneration of the cartilage and a presumed simultaneous regeneration of the posterior horn of the lateral meniscus after BMAC transplantation for a cartilage defect at the lateral tibial and femoral condyle. This report provides the details of a case of an unusual treatment response after a BMAC transplant. This report is the first of its kind to demonstrate a MR image that displays the simultaneous regeneration of the cartilage and meniscus with a differentiation ability of the mesenchymal stem cell to the desired cell lineage.
Recently, mesenchymal stem cell (MSC) tissue engineering techniques have been developed for cartilage regeneration with good clinical outcomes, especially for patients with osteoarthritis (OA) of the knee joints (12). They have also demonstrated the ability to repair a damaged meniscus in knee joints (34). Among these techniques, the bone marrow aspirates concentrate (BMAC) transplantation is a commonly used technique for cartilage regeneration in a knee OA patient (1).
The BMAC transplant is composed of a bone marrow harvest, concentration, and implantation in the cartilage defect sites with a scaffold, and performed under arthroscopic guidance (15). The bone marrow aspiration is usually done upon an iliac crest and the centrifugation of an aspirated bone marrow is essential to the concentrate of the MSCs (5). The final transplantation process involves a placement of a scaffold such as fibrin glue and then the MSC concentration is loaded in the scaffold (25).
The magnetic resonance imaging (MRI) technique has been considered a non-invasive method for evaluating the degree of cartilage regeneration after cartilage repair instead of a second assessment via an arthroscopy (26). After a cartilage regeneration operation, the cartilage defect is filled with regenerated cartilage tissue and the filling degree is categorized as incomplete, complete or hypertrophy (26). The histology of regenerated cartilage is a hyaline-like cartilage, fibrocartilage or mixed hyaline and fibrocartilage (57). The amount of time required for regeneration of cartilage is unknown; although, previous studies have conducted the follow-up MRI between 6 and 34 months (26).
Many studies have reported complete or incomplete filling of the cartilage defect at the follow-up MRI after BMAC transplantation (26). However, only a few cases have demonstrated real MR images of cartilage hypertrophy as a response to a cartilage regeneration technique (6). Moreover, the simultaneous regeneration of a knee cartilage and meniscus has not been previously reported in literature; therefore, a literature review of BMAC transplantation and its relationship to radiology or orthopedic surgery is provided. In this current report, an unusual case of an unexpected regeneration of a damaged meniscus with cartilage hypertrophy at the cartilage defect site is provided along with a literature review.
A 44-year-old man visited our outpatient clinic with right knee pain that commenced 9 years ago and was aggravated after exercise a month ago. Patterns of the knee pain were intermittent and throbbing (numeric rating scale, 5 points). The patient revealed previous surgical history of a partial meniscectomy for the anterior horn of the lateral meniscus in his right knee joint 13 years ago.
A right knee MRI performed at an outside hospital 5 months ago indicated high grade chondral defects at the lateral femoral condyle and the lateral tibial plateau (Fig. 1a) with a complex tear at the posterior horn of the lateral meniscus (Fig. 1b).
Arthroscopic BMAC transplantation was planned for cartilage regeneration and pain reduction in the patient's right knee joint. The arthroscopic view demonstrated a grade IV (of the modified Outerbridge grading system) cartilage defect at the lateral femoral condyle and lateral tibial plateau that was approximately 4 × 4 cm and 2 × 2 cm in size, respectively (Fig. 2a). There were mixed horizontal and radial tears at the posterior horn of the lateral meniscus. During the surgery, a subtotal meniscectomy was initially performed for the posterior horn of the lateral meniscus (Fig. 2a). For BMAC transplantation, a chondroplasty was conducted at the cartilage defect sites of the lateral tibial plateau and lateral femoral condyle with shaving and debridement. Then, a microfracture was conducted at the cartilage defect sites by an arthroscopic awl (Fig. 2b). An orthopedic surgeon then aspirated 60 mL of bone marrow from the right anterior superior iliac spine by a syringe with 10 mL of citrate anticoagulant. Approximately 6 mL of nucleated cell concentrate (NCC) was produced with the aspirated bone marrow using a bone marrow stem concentration system (Biomet Biologics Inc., Warsaw, IN, USA). The NCC was then injected with scaffolds into the microfracture sites which were held with a green plaster (Fig. 2c).
At 4 weeks after BMAC transplantation, the patient exhibited improvement of knee pain (with numerical rating scale of 0 points) and mild limitation in the range of motion (ROM, 0°–100°). At 7 weeks after the operation, a post-operative follow-up MRI was performed. It revealed a healed state of the high grade chondral defects at the lateral femoral condyle and lateral tibial plateau with a hypertrophic regeneration of the cartilage in the lateral tibial condyle (Fig. 3a). Regenerated cartilages displayed relatively iso-signal intensities to the adjacent normal cartilage. Surprisingly, a nearly normal appearance of the posterior horn of the lateral meniscus was also observed on the MRI scans; although, they displayed a diffuse increased signal intensity (SI) (Fig. 3b). However, the patient did not have any symptoms related to a full ROM. Thus, no additional surgeries were conducted.
There are various cartilage regeneration techniques for knee OA patients that can be applied prior to MSC transplantation, including microfracture, autologous chondrocyte transplantation (ACT), and intra-articular stem cell injection (57). Among them, a microfracture is a simple, one-step procedure for cartilage proliferation with marrow stimulation. Another conventional cartilage repair technique is mosaicplasty which is the autologous harvest of an osteochondral graft from a non-weight bearing area and transferred to the primary defect (5). However, these conventional techniques have produced only fair to poor clinical outcomes with inadequate defect fillings and early degeneration (7). Regenerated cartilage by a microfracture or mosaicplasty usually results in a histopathologic finding of a mixture of hyaline and fibrocartilage which is biochemically inferior to native hyaline cartilage (5). ACT has produced better clinical results than a microfracture; however, it has many problems that include donor site morbidity, chondrocyte dedifferentiation, and complexity of the procedure (7). An intra-articular stem cell injection has led to meaningful results for pain relief; but, it also has resulted in no significant outcomes for the regeneration of degenerative changes (27). Recently, MSC transplantation, which is a new technique for overcoming these problems, was developed for cartilage regeneration (27).
The MSC technique for cartilage repair has emerged as a strong biological tool with the potential to differentiate into cells that form mesodermal connective tissues and have the ability to migrate to a trophic site (5). Among the MSCs, bone marrow derived stem cells are commonly used with good chondrogenic potential due to the presence of various progenitor cells and ease of harvest (5). Many clinical studies have shown good clinical outcomes of the BMAC techniques for cartilage repair in patients with knee OA (15). They were recently approved by the Food and Drug Administrations (FDA) (1).
Briefly, the BMAC technique consists of a stem cell harvest followed by a concentration with centrifugation and transplantation of concentrated stem cells into the defect site with a supporting scaffold for better cell survival, proliferation, differentiation, and matrix synthesis (15). The iliac crest is a common site for stem cell harvest by bone marrow aspiration because of its higher mean concentration of MSCs compared to other sites (5). Scaffolds have an important role in providing a stable microenvironment and framework to promote new tissue generation and an optimal location for the implanted sites (5).
MRI is a powerful imaging tool after BMAC transplantation in knee joints since it provides a high resolution and rich image contrast to discriminate articular tissues (26). MRI outcomes also exhibit a good correlation with clinical outcomes after MSC transplantation (2). After the BMAC, the cartilage defect was filled with regenerated cartilage, which is advantageous since the filling degree of the cartilage defect is one of the most important factors for successful cartilage regeneration and good clinical outcomes (26). The different categories include complete filling, incomplete filling, and hypertrophy, which are evaluated by comparing it with the level of the adjacent normal cartilage (26). Another important finding is the SI of the repaired tissue which is compared to the adjacent normal cartilage SI. The regenerated cartilage displays an iso-SI or SI alteration to hyper or hypointensity (26). A SI alteration is related to poor clinical outcomes (2). Other MRI findings of the regenerated cartilage include a surface change of the cartilage and subchondral bone changes related to the edema, granulation tissue and sclerosis (26). If the surface of the regenerated cartilage was not intact or subchondral bone changes are observed, it would be related also to poor clinical outcomes (2).
In previous studies, the histology of the regenerated cartilage after BMAC transplantation, unlike the cases of microfracture or mosaicplasty, is typically hyaline-like tissue (5). MSC-related complications such as excessive bone formation and the development of oncologic cells have been suggested as a theoretical problem. However, most studies did not report such complications with the exception of non-specific joint swelling or pain (1). Although, it is not well recognized yet how the regenerated cartilage will behave or experience pathologic changes over time after BMAC transplantation (2).
The hypertrophy of the regenerated cartilage is based on the inherent hypertrophic properties of MSCs mediated by variable protein markers such as collagen type X (COLX) and vascular endothelial growth factor (VEGF) (8). It is inhibited by other markers including the transforming growth factor beta1 (TGF-β1) (8). Regenerated cartilage hypertrophy has been reported in up to 20–25% cases after ACT (6). Ironically, there are only a few MR images of cartilage hypertrophy after ACT (6). To the best of our knowledge, a MR image of cartilage hypertrophy after BMAC has not been reported yet.
Our case provided MRI scans of unexpected and presumed simultaneous meniscus regeneration with hypertrophic regeneration of the cartilage; however, BMAC transplantation was conducted only at the cartilage defect sites. It was hypothesized that transplanted MSC might have migrated out of the scaffold due to the insufficient ability to hold the MSC concentration. In addition, the posterior horn of the lateral meniscus is anatomically adjacent to the cartilage defect sites of the lateral tibial plateau and lateral femoral condyle in this case, which may facilitate the regeneration of the meniscus. Interestingly, the regenerated meniscus displayed a nearly normal shape with a diffuse increased MR SI compared to the dark SI of the normal meniscus. It preferably resembled the cartilage SI. Unfortunately, we could not obtain the arthroscopic or histologic findings of the regenerated meniscus because the patient did not complain of any symptoms. There are quite a few successful clinical reports of meniscus regeneration for both animals and humans; although MSC techniques for meniscus regeneration have not been clinically applied yet (39). Only two reports have displayed the MRI of the regenerated meniscus in the literature (410). One of the studies provided a MR image of solely the volume expansion of the meniscus (10) while the other study provided only a MR image of the regeneration of the torn meniscus with normal dark SI; however, that study used adipose tissue-derived stem cells with a percutaneous injection instead of BMAC transplantation (4).
To the best of our knowledge, there is no published report of the simultaneous regeneration of cartilage and meniscus after MSC transplantation. In other words, this is the first case providing MR images of the regenerative potential of MSC with the ability to differentiate into two dissimilar tissues. Radiologists and clinicians should be familiar with the concept of MSC techniques and their potential with variable MR findings related to MSC transplantation because these tissue engineering techniques are becoming increasingly popular. MRI evaluation is also becoming common and reasonable for the MSC technique. Further studies should be performed to understand the various MRI findings related to MSC transplantation in the future.
Figures and Tables
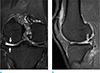 | Fig. 1Right knee MRI of a 44-year-old male presenting with knee pain previously treated at an outside hospital. (a) Coronal fat-saturated T2 weighted image displays high grade cartilage defects (white arrows) in the lateral tibial plateau and lateral femoral condyle. (b) Sagittal fat-saturated proton density weighted image exhibits a complex tear (empty arrow) in the posterior horn of the lateral meniscus. |
 | Fig. 2Arthroscopic finding during the bone marrow aspirates concentrate (BMAC) transplantation. (a) Grade IV cartilage defects at the lateral tibial plateau (empty arrow) and lateral femoral condyle (white arrow) and residual posterior horn of the lateral meniscus (black arrow) after a subtotal meniscectomy are provided. (b) A cartilage defect at the lateral tibial plateau after a microfracture is displayed on an arthroscopic image. (c) BMAC transplantation into the lateral tibial plateau with a scaffold is performed. |
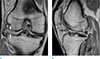 | Fig. 3Follow-up right knee MRI of the patient at 7 weeks after the operation. (a) Coronal proton density weighted image displays hypertrophic regeneration of the cartilage (white arrows) at the lateral tibial plateau and the near complete regeneration of the cartilage at the lateral femoral condyle (white arrows). (b) The sagittal proton density weighted image exhibits a nearly normal-shaped posterior horn of the lateral meniscus (empty arrow) though displays a diffuse increased signal intensity. |
References
1. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016; 4:2325967115625481.
2. Kim YS, Choi YJ, Lee SW, et al. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthritis Cartilage. 2016; 24:237–245.
3. Pak J, Lee JH, Park KS, Jeon JH, Lee SH. Potential use of mesenchymal stem cells in human meniscal repair: current insights. Open Access J Sports Med. 2017; 8:33–38.
4. Pak J, Lee JH, Lee SH. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. Biomed Res Int. 2014; 2014:436029.
5. Holton J, Imam MA, Snow M. Bone marrow aspirate in the treatment of chondral injuries. Front Surg. 2016; 3:33.
6. Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004; 52:310–319.
7. Freitag J, Bates D, Boyd R, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet Disord. 2016; 17:230.
8. Chen S, Fu P, Cong R, Wu H, Pei M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015; 2:76–95.
9. Yu H, Adesida AB, Jomha NM. Meniscus repair using mesenchymal stem cells - a comprehensive review. Stem Cell Res Ther. 2015; 6:86.
10. Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses. 2008; 71:900–908.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download