Abstract
A 7-year-old boy, diagnosed with an arachnoid cyst and subdural effusion on initial MRI, was admitted with left limb weakness and no history of head trauma. A subsequent follow-up MRI showed different stages of hematoma within multilayered enhancing membranes and in the arachnoid cyst, which was separated by the cerebrospinal fluid cleft. Craniotomy and fenestration of the cyst wall and hematoma removal were performed. The patient was diagnosed as a having a hemorrhagic rupture of an arachnoid cyst into the intradural space, probably via some one-way valve-like defect, based on the MRI and surgical findings. The MRI features and possible mechanism of this rare disease are discussed within the literature review.
Arachnoid cysts are developmental lesions, which are extracerebral, intra-arachnoidal cerebrospinal fluid (CSF) collections that are a common incidental finding on routine brain imaging (1). While the majority of these lesions can present as an asymptomatic mass, they can become symptomatic upon cyst enlargement or hemorrhage (2). Arachnoid cysts are occasionally associated with subdural effusion, which is generally thought to result from tearing of the outer wall of the arachnoid cyst after head injury (3). However, the precise mechanism for the formation of an arachnoid cyst with subdural lesion is not known. MRI of the patient showed different stages of hematoma within multilayered enhancing membranes, suggesting an intradural space arachnoid cyst. We present the possible mechanism of this rare disease with literature review, which provides a comprehensive understanding for the pathogenesis of an arachnoid cyst with dural lesion.
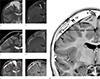
A 7-year-old boy with headache and vomiting for several days was admitted via the emergency room. The patient had no focal neurological deficits on initial presentation. There had been no history of birth trauma, head trauma or external wound in recent years. MRI showed an arachnoid cyst and subdural hygroma on the right frontoparietal vertex (Fig. 1a, b), suggesting that there was no acute hemorrhage due to head trauma. The patient was to be observed without surgical intervention. However, he revisited after three months due to continuous headache and sudden onset of left lower limb weakness. Subsequent follow-up MR imaging revealed several layers of fluid collection that appeared to evolve by splitting the enhanced, thickened pachymeninges and intracystic hemorrhage, regarded as an intracystic hematoma of the arachnoid cyst (Fig. 1c, d, g).
Craniotomy and fenestration of the cyst wall and hematoma removal were performed. During surgery, there was no evidence of superior sagittal sinus laceration. There were inner and outer membranes of chronic subdural hematoma and similarly colored intracystic hematoma was also found. Pathologic examination revealed three layers of dura mater and a thin innermost cyst wall. Postoperatively, the patient recovered from hemiparesis. Postoperative follow-up MRI showed contour bulging of the innermost cystic lesion after removal of hematoma and blood filling the cystic lesion (Fig. 1e, f). On follow up after two weeks, the patient had no specific complaints or neurologic deficit and was discharged uneventfully.
In this case, the anatomical relationship between the arachnoid membrane and three layers of dura mater was clearly demonstrated on the MRI. As shown in the baseline MRI, a cystic lesion without wall enhancement was located above the brain and below the CSF space. As arachnoid mater does not generally show contrast enhancement, the deepest located cystic lesion was regarded as an arachnoid cyst. According to the histological findings, the structure of the arachnoid cyst consisted of a very thick layer of collagen that did not traverse the trabecular processes, which suggests that an arachnoid cyst (a type of developmental anomaly) is formed when primitive arachnoid membrane is split or duplicated (4).
After rupture of the arachnoid cyst, three enhancing layers covered the arachnoid cyst and CSF space on contrast enhanced T1-weighted images, which is thought to be dura mater. In addition, the enhancing dura mater covered the hematoma with low signal intensity on T2-weighted images, which was not subdural, but actually intradural. The usual chronic subdural hematoma is thought to be in a chronic “intradural” location and has been described clinically and anatomically (5). Pathologically, the dural layers are composed of three layers: dural border cells, meningeal dura and periosteal dura. The intradural space is only a potential space and becomes a physical reality by a cleavage opening the weakest structural plate of the pachymeninges. This space is technically intradural and not subdural in location and is created by the junctional disruption of the dural border cell layer from the deep pachymeninges and subsequent acquisition of a space-occupying hematoma (6).
Although the rupture of an arachnoid cyst can occur spontaneously, increased pressure from the arachnoid cyst may result in a rupture if there is also accompanying vascular disruption (7). According to electron microscopic data of human bridging veins, the subarachnoid portion of the bridging vein has a wall of uniform thickness and is embedded into the loose tissue of the arachnoid trabeculae. However, certain portions of this vein have no abutting arachnoid trabecular tissue reinforcing the wall from the outside (8). The wall of an arachnoid cyst is lined by mesothelium and it contains unsupported vessels. An arachnoid cyst with intracystic bleeding has previously been related to unsupported vessels (9). Here we showed a tubular structure of high signal intensity on T1-WI within an arachnoid cyst, which implies a torn vessel.
We hypothesized that this patient already had contact between the arachnoid cyst, intradural space and chronic subdural effusion. With fluid production from the cyst wall and a one-way valve flow of CSF, the size of the arachnoid cyst might have increased. By increasing the cerebrocranial disproportionately, an unsupported vessel in the arachnoid cyst was stretched above the tensile limit. The blood leaking from the ruptured vessel first filled the arachnoid cyst and then overflowed through the intradural space. As the pressure in the intradural space increased, the size of the arachnoid cyst was unchanged and the increased pressure of the cyst stopped further bleeding. Likely due to one-way valve flow, the signal intensity of the arachnoid cyst looked like a subacute stage, whereas the signal intensity of the intradural space was late stage. Spontaneous regression or disappearances of arachnoid cysts have been reported in rare cases and contact between the arachnoid cyst and subdural space was proposed as the mechanism of the regression (10). However, the case presented here showed a communication between the arachnoid cyst and “intradural” space with a non-traumatic hemorrhagic complication. To the best of our knowledge, this is the first report demonstrating communication between an arachnoid cyst and the intradural space with non-traumatic hemorrhage.
In summary, we report a case of non-traumatic hemorrhagic rupture of an arachnoid cyst into the intradural space, which was well distinguished from the subdural space on MRI. From this case we can understand the precise anatomical relationship between the arachnoid membrane and three layers of the dura mater. In regard to arachnoid cysts in a clinical environment, we should consider that potential contact between an arachnoid cyst and the intradural space could exist and spontaneous hemorrhaging into the intradural space could occur with concurrent neurologic deficits.
Figures and Tables
Fig. 1
A 7-year-old boy with a hemorrhagic rupture of an arachnoid cyst into the intradural space. T2-weighted image (a) shows a cystic lesion covering the right frontal lobe. A low signal intensity linear structure (black arrow) divides the cystic space, which shows contrast enhancement (white arrow) on contrast enhanced T1-weighted image (b). Follow-up MRI three months later (c, d) shows three different cystic spaces. The high signal intensity tubular structure (black arrows) implies a torn vessel. The blood leaking from the ruptured vessel first filled the arachnoid cyst and then overflowed through the intradural space. Postoperative MRI (e, f) shows contour-bulging of the innermost cystic lesion (black arrow) after removal of hematoma and blood-filled cystic lesion. Schema using inverse imaging of T2-weighted image (g) shows three separated cysts (c1: arachnoid cyst; c2: intradural cyst; c3: subdural space), which are separated by a cerebrospinal fluid cleft and multilayer membranes (arrowhead: meningeal dura; double arrowheads: intermediate dura; triple arrowheads: periosteal dura). Tubular structure (black arrow) within the arachnoid cyst (c1) suggests a torn, unsupported vessel. Signal intensity of the cyst suggests one-way valve flow from c1 to c2 and bidirectional communication between c1 and c2.
References
1. Galassi E, Piazza G, Gaist G, Frank F. Arachnoid cysts of the middle cranial fossa: a clinical and radiological study of 25 cases treated surgically. Surg Neurol. 1980; 14:211–219.
2. Iaconetta G, Esposito M, Maiuri F, Cappabianca P. Arachnoid cyst with intracystic haemorrhage and subdural haematoma: case report and literature review. Neurol Sci. 2006; 26:451–455.
3. Gelabert-Gonzalez M, Fernandez-Villa J, Cutrin-Prieto J, Garcia Allut A, Martinez-Rumbo R. Arachnoid cyst rupture with subdural hygroma: report of three cases and literature review. Childs Nerv Syst. 2002; 18:609–613.
4. Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981; 40:61–83.
5. Domenicucci M, Strzelecki JW, Delfini R. Acute post-traumatic subdural hematomas: “intradural” computed tomographic appearance as a favorable prognostic factor. Neurosurgery. 1998; 42:51–55.
6. Haines DE, Harkey HL, al-Mefty O. The “subdural” space: a new look at an outdated concept. Neurosurgery. 1993; 32:111–120.
7. Huang D, Abe T, Kojima K, et al. Intracystic hemorrhage of the middle fossa arachnoid cyst and subdural hematoma caused by ruptured middle cerebral artery aneurysm. AJNR Am J Neuroradiol. 1999; 20:1284–1286.
8. Yamashima T, Friede RL. Why do bridging veins rupture into the virtual subdural space? J Neurol Neurosurg Psychiatry. 1984; 47:121–127.
9. Eustace S, Toland J, Stack J. CT and MRI of arachnoid cyst with complicating intracystic and subdural haemorrhage. J Comput Assist Tomogr. 1992; 16:995–997.
10. Yilmaz C, Cetinalp E, Caner H, Altinors N. Dissapearance of arachnoid cyst after rupturing into subdural space. Acta Neurochir (Wien). 2007; 149:731–733.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download