Abstract
Schwannomas are benign nerve sheath tumors that are typically located in soft tissue. Occasionally, schwannomas involve osseous structures. These intraosseous schwannomas are generally benign neoplasms that account for less than 0.2% of primary bone tumors. Schwannomas are very rarely observed in long bones. We present a case of a schwannoma affecting the proximal femur with a coincident subchondral fracture of the femoral head. A 38-year-old-male presented with left hip pain without deteriorating locomotor function. Plain film radiographs displayed a lobulating contoured lesion within the intertrochanteric portion of the femur. The magnetic resonance imaging (MRI) scans showed a tumor occupying the intertrochanteric region. Diffuse bone marrow edema, especially in the subchondral and head portions of the femur that was possibly due to the subchondral insufficiency fracture was also noted. The lesion was surgically excised and bone grafting was performed. Histologically, there was diffuse infiltrative growth of the elongated, wavy, and tapered cells with collagen fibers, which are findings that are characteristic of intraosseous schwannoma. Although very rare, intraosseous schwannoma should be included in the differential diagnosis of radiographically benign-appearing, non-aggressive lesions arising in the femur. The concomitant subchondral fracture of the femoral head confounded the correct diagnosis of intraosseous schwannoma in this case.
In general, schwannomas are benign neurogenic tumors that occur primarily in soft tissues. Very few schwannomas in intraosseous regions have been reported, and they account for less than 0.2% of primary bone tumors (1). The clinicopathological features or pathophysiologic mechanisms of intraosseous schwannomas are still unclear. The radiologic findings are non-specific; these tumors manifest as well-defined osteolytic lesions with a thin peripheral sclerotic rim. In all of the cases that we reviewed in the related literature, the diagnosis of intraosseous schwannoma was not made until after the histopathologic examination of tissues obtained surgically or from tissue sampling due to the rarity of osseous involvement. We encountered a case of an intraosseous schwannoma in a patient with a concomitant subchondral fracture of the femoral head.
A 38-year-old male was referred to our hospital due to non-remitting pain in his left hip and groin area for duration of 2 months. He was in good health and had no relevant trauma, infections, or underlying systemic diseases. A plain hip anteroposterior (AP) radiograph displayed a lobulating contoured lesion in the intertrochanteric portion of the left femur (Fig. 1). Computed tomography (CT) exhibited an osteolytic lesion with a relatively indistinct margin and subtle sclerotic rim involving the intertrochanteric region of the left femur. There was endosteal erosion at the medial cortex of the femur. No cortical disruption of the femur was observed (Fig. 2a). These findings are concordant with a benign, rather than a malignant, bone tumor. There was a focal horizontal cortical deformity of the femoral head that was suggestive of a subchondral insufficiency fracture (Fig. 2b). A technetium-99m whole-body bone scintigraphy demonstrated marked radiotracer uptake not only at the affected site at the intertrochanteric region, but also at the left femoral head (Fig. 3).
On the magnetic resonance imaging (MRI) scans, the mass manifested as a lobulating contoured tumor with an isointense signal intensity to the skeletal muscle on T1-weighted images and a subtle high signal intensity on T2-weighted images. The post-contrast T1-weighted fat-saturated sequences displayed a relatively homogeneous enhancement. The mass was determined to be 3.5 × 2.7 × 2.0 cm in size. Regardless of the presence of the tumor, the normal bone marrow signal intensity was noted around the mass at the femur shaft. Uninvolved parts of the femur and pelvic bones had normal marrow signal intensities. There was no periostitis, extraosseous tumor spread, adjacent soft tissue mass formation, or soft tissue edema. There were no enlarged regional lymph nodes. Therefore, the lesion was suggested to be a benign bone tumor entity, and radiographic differential diagnosis included hemangioma, enchondroma, and eosinophilic granuloma. However, the possibility of malignancy could not be excluded. Another remarkable finding was the diffuse bone marrow edema primarily in the femoral head which extended to the neck portion. This non-specific bone marrow edema was possibly due to the subchondral insufficiency fracture. The patient underwent an intra-lesional tumoral curettage and autologous bone graft filling. Based on the CT, MRI and whole-body bone scintigraphy findings, we hypothesized that the bone marrow edema and radioactive tracer accumulation in the femoral head and neck were due to a subchondral fracture of the femoral head rather than the tumor (Fig. 4). A histologic analysis revealed that the lesion had a diffusely infiltrative growth between the trabecular bones. There were areas of the spindle-shaped cells with an abortive palisading appearance and a scanty amount of eosinophilic material that simulated a well-formed Verocay body. The immunohistochemical staining for S-100 proteins was positive (Fig. 5).
Schwannomas are known as neurilemmomas, neurinomas, and perineural fibroblastomas. A schwannoma is a benign neurogenic tumor that consists of Schwann cells, which generate the peripheral nerve sheath that covers the myelinated nerve fibers (2). About 25–40% of schwannomas are seen in the skin or subcutaneous tissue of the head and neck. Others have been found on the flexor surfaces of the extremities. In addition, schwannomas are very rare in individuals with underlying neurocutaneous syndrome such as neurofibromatosis.
In the past, researchers have used the terms “intraosseous schwannoma” and “intraosseous neurofibroma” interchangeably; but they are now distinguishable. Schwannomas typically arise in association with a nerve that enters the bone through a nutrient canal. Intraosseous schwannomas are rarely seen outside of the primary diagnosis of neurofibromatosis type I or von Recklinghausen's disease (3). Intraosseously-located schwannomas are extremely rare, and there is scant literature documenting femur involvement. Because of its rarity, an intraosseous schwannoma is therefore not generally considered during the differential diagnosis of tumorous lesions of the femur. Only six cases of intraosseous schwannoma involving the femur have been reported in the medical literature (4).
Schwannoma involvement of the bone was postulated to occur through three mechanisms: 1) origination from the bone, 2) growth within the nutrient canal and secondary widening or penetration of the medullary canal, or 3) an initial extraosseous location, followed by subsequent erosion of the bone. The second type is the most common mechanism by which intraosseous schwannomas affect long bones (5). In most cases, these schwannomas display the characteristics of benign bone tumor entities. They present as a slow-growing, painless mass and are asymptomatic in 25% of cases. Other clinical manifestations include non-specific local pain or local swelling with pain when combined with a secondary infection. In rare cases, a microfracture or pathological fracture can occur (6).
The radiologic features of an intraosseous schwannoma are consistent with those of benign bone tumors. The plain radiograph findings were as follows: 1) a well-circumscribed osteolytic lesion possessing a sclerotic margin, 2) a cyst-like lesion with a unilocular or multilocular form, mimicking a simple bone cyst, 3) protrusion or erosion into the adjacent bone, 4) absence of central calcification, 5) thinning of the cortex, and 6) rarely concomitant periosteal reactions. Osseous lesions with similar characteristics are simple bone cysts, aneurysmal bone cysts, non-ossifying fibromas, benign fibrous histiocytomas, desmoplastic fibromas, fibrous dysplasia, chondromyxoid fibromas, and enchondromas. Because the findings for intraosseous schwannomas are non-specific, plain radiographs are not sufficient for precise diagnosis (7). On the MRI scans, these tumors exhibited an isointense low signal intensity compared to the muscle on T1-weighted images, high signal intensity on T2-weighted images, and contrast enhancement with gadolinium. It seems that massive bone marrow edema in the femoral head in our case was possibly due to the existence of a subchondral fracture, and not the tumor itself. The shape can be another key to differential diagnosis. In cases of paraspinal schwannomas, a dumbbell shape is a typical finding because of the tumor's tendency to extend into an enlarged neural foramen. The recognition of a fusiform lesion continuous with the nerve can be another strong indicator of a peripheral nerve sheath tumor such as a schwannoma (8).
In many cases, however, diagnosis is not even suspected until the histological appearance is evaluated after a biopsy or surgical resection. Due to the benign nature of these tumors, surgical curettage and bone grafting ensure a good prognosis. Histopathologically, schwannomas are usually composed of two components. One component is Antoni A type tissue, which consists of highly ordered cellular components with degenerative changes and variable admixtures of compact spindled cells. The second component is Antoni B type tissue, which is hypocellular, microcystic tissue rich in macrophages and collagen fibers within a loose myxoid component (9). In Antoni A type tissue, homogeneous acellular zones with nuclear fencing known as Verocay bodies are sometimes noted. Verocay bodies and nuclear fencing are associated with nerve cell differentiation. The Antoni B region consists of randomly organized tissue with microcysts. Intraosseous schwannoma and soft tissue schwannoma share similar histologic features. However, the former shows a higher degree of cellularity with slight fencing and inadequate Verocay bodies. Positive immunoreactivity for S-100 proteins is indicative of soft tissue and peripheral nervous system tumors including Schwann cell origin tumors.
This report highlights the importance of considering the possibility of intraosseous schwannoma in the differential diagnosis of osseous tumors when presented with a benign-appearing femoral bone lesion. Because the clinical course and the radiographic appearance of intraosseous schwannoma are not always typical, the diagnosis can be feasible only after histologic examination. In our case, concomitant bone marrow edema was responsible for our original misdiagnosis. In our case, the focal horizontal cortical deformity of the femoral head represented a subchondral fracture of the femoral head. We deduced the subchondral fracture as the culprit of the diffuse bone marrow edema at the proximal femur. The possibility of transient osteoporosis and transient bone marrow edema syndrome should be considered; therefore, confirmation and follow-up studies are needed. The presence of the associated marrow edema from the fracture probably obscured the true imaging characteristics of the mass. Despite the fact that the imaging findings of the mass showed benign features, the lesion was curetted and excised which yielded the pathologic diagnosis for the intraosseous schwannoma.
In conclusion, we reported a case of intraosseous schwannoma in the left proximal femur. The MRI and CT scans demonstrated a lobulating contoured mass with secondary widening and thinning of the adjacent medullary canal. A concomitant subchondral fracture of the femoral head was another feature that confounded the correct diagnosis of intraosseous schwannoma. For precise diagnosis and to avoid unnecessary treatment, surgical tissue sampling is mandatory.
Figures and Tables
Fig. 1
An anteroposterior radiograph of the hip joint. This image displays ill-defined, osteolytic changes. A lobulating contoured intramedullary expansile mass (white arrow) can be seen in the proximal femur. No definite sclerotic margin, cortical thinning, or swelling of the overlying superficial cortex is visible.
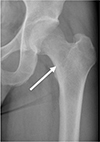
Fig. 2
Computed tomography (CT) scan of left femur. (a) The coronal image displays an intramedullary mass in the intertrochanteric region of the left femur (white arrow). (b) The sagittal image exhibits an indistinct radiolucent rim-like structure (white arrows) with horizontal direction at the femoral head, which is suggestive of a subchondral insufficiency fracture.
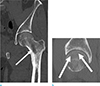
Fig. 3
Technetium-99m whole-body bone scintigraphy. Diffuse increased uptake throughout the left femoral head and intertrochanteric region is noted.
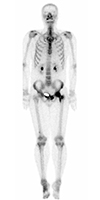
Fig. 4
Preoperative hip MRI. (a) Coronal fat-suppressed T2-weighted image displays a lobulating contoured mass (thick arrow) with a homogeneous nature and the absence of perilesional soft tissue or endosteal edema. Note the diffuse bone marrow edema, especially in the femoral head (thin arrows). (b) On the coronal T1-weighted image, the mass (thick arrow) exhibits an isointense signal intensity to the muscle. (c) On the coronal T2-weighted image, the mass exhibits a slight hyperintense relative to the skeletal muscle (thick arrow). (d) On the post-contrast fat-suppressed coronal T1-weighted image, the mass displays homogeneous enhancement (thick arrow) which indicates its solid nature. The adjacent endosteal bone and soft tissues are normal. No bone marrow edema or contrast enhancement in the femoral shaft adjacent or just inferior to the tumor was noted. The prominent marrow edema with mild contrast enhancement in the head portion of the femur (thin arrows) was believed to be due to the subchondral fracture, not the tumorous lesion itself.
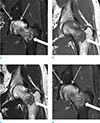
Fig. 5
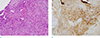
Microscopic examination of the local excised specimen. (a) Photomicrograph of a histologic specimen of schwannoma. Pathologic specimen (original magnification, × 400; Hematoxylin-Eosin stain) displays infiltrative growth of spindle cells forming abortive Verocay bodies (arrows) which have the characteristic findings of wavy, tightly organized, nuclear palisades with mild lymphocytic infiltration. (b) The brown staining was found in the cytoplasm, S-100 protein was positively expressed in the immunohistochemical staining (original magnification, × 400; S-100 stain).
References
1. Bansal AK, Bindal R, Shetty DC, Dua M. Rare occurrence of intraosseous schwannoma in a young child, its review and its pathogenesis. J Oral Maxillofac Pathol. 2012; 16:91–96.
2. Simsek HO, Aksoy MC, Can C, Baykul T. Intraosseous schwannoma of the mandible. Int J Exper Dent Sci. 2012; 1:48–50.
3. Sorensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med. 1986; 314:1010–1015.
4. Hoshi M, Takada J, Oebisu N, Nakamura H. Intraosseous schwannoma of the proximal femur. Asia Pac J Clin Oncol. 2012; 8:e29–e33.
5. Gine J, Calmet J, Sirvent JJ, Domenech S. Intraosseous neurilemmoma of the radius: a case report. J Hand Surg Am. 2000; 25:365–369.
6. Suzuki K, Yasuda T, Watanabe K, Kanamori M, Kimura T. Association between intraosseous schwannoma occurrence and the position of the intraosseous nutrient vessel: A case report. Oncol Lett. 2016; 11:3185–3188.
7. Vora RA, Mintz DN, Athanasian EA. Intraosseous schwannoma of the metacarpal. Skeletal Radiol. 2000; 29:224–226.
8. Imaizumi A, Kodama S, Sakamoto J, et al. Imaging findings of benign peripheral nerve sheath tumor in jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116:369–376.
9. Weiss SW, Goldblum JR, Folpe AL. Enzinger and Weiss's soft tissue tumors. Philadelphia, USA: Elsevier Health Sciences;2007.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download