Abstract
Purpose
To evaluate the diagnostic performance of diffusion-weighted steady-state free precession (DW-SSFP) in comparison to diffusion-weighted echo-planar imaging (DW-EPI) for differentiating the neoplastic and benign osteoporotic vertebral compression fractures.
Materials and Methods
The subjects were 40 patients with recent vertebral compression fractures but no history of vertebroplasty, spine operation, or chemotherapy. They had received 3-Tesla (T) spine magnetic resonance imaging (MRI), including both DW-SSFP and DW-EPI sequences. The 40 patients included 20 with neoplastic vertebral fracture and 20 with benign osteoporotic vertebral fracture. In each fracture lesion, we obtained the signal intensity normalized by the signal intensity of normal bone marrow (SI norm) on DW-SSFP and the apparent diffusion coefficient (ADC) on DW-EPI. The correlation between the SI norm and the ADC in each lesion was analyzed using linear regression. The optimal cut-off values for the diagnosis of neoplastic fracture were determined in each sequence using Youden's J statistics and receiver operating characteristic curve analyses.
Results
In the neoplastic fracture, the median SI norm on DW-SSFP was higher and the median ADC on DW-EPI was lower than the benign osteoporotic fracture (5.24 vs. 1.30, P = 0.032, and 0.86 vs. 1.48, P = 0.041, respectively). Inverse linear correlations were evident between SI norm and ADC in both neoplastic and benign osteoporotic fractures (r = −0.45 and −0.61, respectively). The optimal cut-off values for diagnosis of neoplastic fracture were SI norm of 3.0 in DW-SSFP with the sensitivity and specificity of 90.4% (95% confidence interval [CI]: 81.0–99.0) and 95.3% (95% CI: 90.0–100.0), respectively, and ADC of 1.3 in DW-EPI with the sensitivity and specificity of 90.5% (95% CI: 80.0–100.0) and 70.4% (95% CI: 60.0–80.0), respectively.
In patients with vertebral compression fracture, the differential diagnosis between neoplastic and benign osteoporotic fractures is important to provide proper early therapeutic intervention and improve prognosis and quality of life (1). As pathologic confirmation for the differential diagnosis is not always feasible due to advanced age and general condition, the usual practice is generally based on the clinical information and findings of spine magnetic resonance imaging (MRI) (123). In conventional MRI sequences, such as T1- or T2-weighted images, morphologic features like the degree and pattern of bone marrow replacement, multiplicity of lesions, presence of paravertebral soft tissue masses, infiltration of posterior elements of the vertebrae, and presence of a fracture line are commonly evaluated for the differential diagnosis (12345678). However, accurate differential diagnosis between these fractures can be challenging since there are overlapping morphological features and frequent mimickers (34567891011).
Diffusion-weighted (DW) imaging has emerged to help the differential diagnosis by detecting signal changes at the fractured vertebrae (5610). DW echo-planar imaging (DW-EPI) sequence is the accepted differential diagnostic tool. However, DW steady-state free precession (DW-SSFP) sequence has been considered relatively inappropriate for clinical application. Although DW-SSFP has strengths in reflecting the molecular diffusion, T2* relaxation, and clear anatomical information, its vulnerability to motion and susceptibility artifacts, and T2 shine-through effect have challenged its clinical use (567912). However, with the technical advances of MRI hardware and development of the 3-Tesla (T) system, DW-SSFP has a better signal-to-noise ratio and higher resolution, which provides more accurate anatomical information by overcoming motion artifacts and susceptibility artifacts, as well as faster scanning time (12).
Several studies have recently explored the utility of DW-SSFP for the differential diagnosis of vertebral fractures (1013). Baur et al. (10) used visual estimation and semi-quantification of the signal intensities to observe significantly increased DW-SSFP signal intensity on the DW-SSFP in neoplastic fractures compared to benign osteoporotic fractures. Bhugaloo et al. (4) demonstrated that DW-SSFP has high positive and negative predictive values for the diagnosis of neoplastic and benign osteoporotic fractures. However, Biffar et al. (14) argued that signals from the DW-SSFP are not accurate and possibly misleading, and therefore should not be used for the differential diagnosis of vertebral fractures.
To the best of our knowledge, no study has explored the relationship between DW-SSFP and DW-EPI in the differential diagnosis of the neoplastic and the benign osteoporotic vertebral fractures using the 3-T MRI. Thus, we evaluate the diagnostic performance of DW-SSFP for differentiating neoplastic vertebral fractures from benign osteoporotic vertebral fractures in comparison with DW-EPI.
This retrospective study was approved by our Institutional Review Board and the written informed consent for MRI was obtained from all patients prior to the examination. Between September 2013 and April 2015, 323 patients underwent 3T spine MRI, including both DW-SSFP and DW-EPI sequences. Of these, we included 157 patients who had recent vertebral fractures on MRI. Of the 157 patients, 117 patients with factors that could influence the MR signal intensities, such as history of vertebroplasty, spine operation, chemotherapy, or radiotherapy or with suboptimal image quality were excluded. The remaining 40 patients comprised the study subjects.
MRI scans were acquired using the 3T unit (MAGNETOM Skyra; Siemens Medical Solutions, Erlangen, Germany). The protocols included DW-SSFP, DW-EPI, and T1- and T2-weighted images. DW-SSFP was acquired with the following parameters: diffusion moment, 90 to 150 mT/m(*)msec; repetition time (TR), 13.93 msec; echo time (TE), 3.99 msec; and flip angle, 35 degrees. The estimated time for DW-SSFP image acquisition was 2 min ± 10 sec per patient. Sagittal image of DW-SSFP was obtained with field of view (FOV) of 150 × 300 and slice thickness of 3 mm. For the DW-EPI, we utilized the monopolar diffusion scheme with b-values of 0, 400, 800, 1000, and 1400 sec/mm2, TR, 3200 msec and TE, 65 msec. Sagittal image of DW-EPI was obtained with FOV of 150 × 300 and slice thickness of 4 mm. Multiple b-values, including 1400, were used to minimize the T2 shine-through effects in calculating the apparent diffusion coefficient (ADC). The estimated time for DW-EPI image acquisition was 4 min ± 20 sec per patient. T1-weighted axial and sagittal images were acquired using the following parameters: TR, 570 msec; TE, 10 msec; slice thickness, 3 mm (sagittal orientation) and 5 mm (axial orientation); and FOV, 320 × 320. T2-weighted axial and sagittal images were acquired using the following parameters: TR, 2990 msec; TE, 46 msec; slice thickness, 3 mm (sagittal orientation) and 5 mm (axial orientation); and FOV, 448 × 308.
The patients were divided into the neoplastic fracture group and the benign osteoporotic fracture group, based on the clinical information, spine MRI findings, and imaging follow-up. Patients in the neoplastic fracture group had a medical history of extra-skeletal malignancy; vertebral compression fracture either with mass replacing the bone marrow, mass involving the posterior elements of the vertebrae, paravertebral soft tissue mass, or multiple other spinal metastases on the T1- and T2-weighted images; and progression of the vertebral mass in the fracture site on the follow-up imaging (size increase or new metastatic lesion in the different vertebrae). The fracture lesions of the patients in the neoplastic fracture group were defined as the neoplastic fractures. These lesions were evaluated before the start of chemotherapy or radiotherapy, which may influence signal intensity of the bone marrow. Imaging follow-up was done with cross-sectional studies, such as MRI, computed tomography, or positron emission tomography-computed tomography. The mean duration of imaging follow-up was 6 months with a range of 3 to 12 months in the neoplastic fracture group.
The mean age of the 20 patients in the neoplastic fracture group (10 males and 10 females) was 61.6 years (range 35 to 85 years). The patients had lung cancer (n = 7), breast cancer (n = 4), multiple myeloma (n = 2), musculoskeletal sarcoma (n = 2), esophageal cancer (n = 1), hepatocellular carcinoma (n = 1), prostate cancer (n = 1), renal cell carcinoma (n = 1), or malignant thymoma (n = 1). Within the neoplastic fracture group, there were no statistically significant differences in bone marrow signal intensities on both DW-SSFP and DW-EPI (P = 0.300, and P = 0.220, respectively).
In patients who had multiple metastases or mass-forming lesions in the spine, especially in patients with multiple myeloma and esophageal cancer, only recent vertebral fractures were evaluated. Old vertebral fractures with sclerotic or fatty changes were excluded. As no patients had multiple recent vertebral fractures, only one recent vertebral fracture per patient was included in the analysis.
Patients in the benign osteoporotic fracture group had osteoporosis based on bone mineral density, negative tumor markers, and clinical history of trauma or hormonal imbalance, which increases the risk of osteoporotic fracture. Vertebral compression fractures in this group showed linear bands on the T1- and T2-weighted images with normal background bone marrow signal intensity of the vertebral body. In addition, their fracture lesions were stable during the imaging follow-up of 6 months or longer. The fracture lesions of the patients in the benign osteoporotic fracture group were defined as the benign osteoporotic fractures. As no patients had multiple recent vertebral fractures, only one recent vertebral fracture per patient was included in the analysis. A total of 20 osteoporotic vertebral fracture patients were included in our study.
Four radiologists with various years of experience in spine MRI performed the qualitative and quantitative measurements at the fracture sites. Consensus was reached if there was any discrepancy in the detection of target lesion, especially when there was no definitely delineated fracture line on DW images. Regions of interest (ROIs) were drawn on both DW-SSFP and DW-EPI at the sites correlating to the target lesion detected on T1- and T2-weighted images. Circular ROIs with areas of less than 10 mm2 were consistently applied at the center of the target lesion. To minimize the inter-observer variability, we calculated the means of the quantitative measurement by the four readers and performed reassessment if there was an extreme discrepancy among the readers. The median areas of the ROI for neoplastic and benign osteoporotic fractures were 3.3 mm2 and 3.5 mm2, respectively. For the DW-SSFP, the signal intensity of the target lesion was normalized by the signal intensity of the normal bone marrow according to the following formula: SI norm = ([signal intensity of the target lesion] − [signal intensity of the normal bone marrow])/(signal intensity of the normal bone marrow). For the DW-EPI, the ADC value was measured at the target lesion (Figs. 1, 2).
The measured SI norm and ADC at the neoplastic fracture site were compared to those at the benign osteoporotic fracture site using Mann-Whitney U test. To evaluate the correlation between the SI norm and ADC, linear regression analysis was conducted in each group. The optimal cut-off values for the diagnosis of neoplastic fracture were calculated in each sequence using Youden's J statistics and receiver operating characteristic (ROC) curve analyses along with the sensitivity and specificity. Statistical analyses were performed using SPSS Statistics version 18.0 (IBM Corporation, Armonk, NY, USA). A two-sided P-value < 0.05 was considered to indicate statistical significance.
In the neoplastic fractures, the median SI norm on DW-SSFP was higher and the median ADC on DW-EPI was lower than the benign osteoporotic fracture (5.24, range 2.31–11.50 vs. 1.30 range, 0.10–3.12; P = 0.032, and 0.86, range 0.32–1.35 vs. 1.48, range 1.05–1.86; P = 0.041, respectively) (Fig. 3). All of the neoplastic fracture group had SI norms higher than 3.0, while the majority of the benign osteoporotic fracture group had SI norms lower than 3.0. In addition, all of neoplastic fracture group had ADC values lower than 1.0, while the majority of the benign osteoporotic fracture group had ADC values higher than 1.0.
Inverse linear correlations were demonstrated between SI norm and ADC in both neoplastic and benign osteoporotic fractures, with weak and moderate relationships (correlation coefficients, r = −0.45; P = 0.012 for neoplastic fracture and r = −0.61; P = 0.013 for benign fracture) (Fig. 4).
The optimal cut-off value for the diagnosis of neoplastic fracture in DW-SSFP was SI norm of 3.0 with the sensitivity and specificity of 90.4% (95% confidence interval [CI]: 81.0–99.0) and 95.3% (95% CI: 90.0–100.0), respectively. The optimal cut-off value in DW-EPI was ADC of 1.3 with the sensitivity and specificity of 90.5 (95% CI: 80.0–100.0) and 70.4 (95% CI: 60.0–80.0), respectively (Fig. 5). The areas under the ROC curve were 0.82 for DW-SSFP (P = 0.013) and 0.71 for DW-EPI (P = 0.027). There was no statistically significant difference between the two areas under the curve (P = 0.250).
This is the first study using 3-T MRI to explore the diagnostic performance of DW-SSFP in differentiating neoplastic and benign osteoporotic fractures in comparison to that of DW-EPI. Previous studies used 1.5-T MRI to investigate the sensitivity and specificity of the DW-EPI and DW-SSFP in determination of the neoplastic vertebral fracture from the benign fracture. Even so, there were no comparisons between the two sequences. Our study is unique in that we compared the diagnostic accuracy of DW-SSFP in comparison to DW-EPI using 3-T MRI.
Since its development, DW-SSFP has been refined and now features a high signal-to-noise ratio and contrast-to-noise ratio, along with better MR hardware (15). Unlike the DW-EPI, the signal from the DW-SSFP is a weighted combination of T2-weighted spine echo and multiple stimulated echo pathways with unique b-values. Its multiple TR and long effective TE, combined with rephrasing correct pulse, which eliminates T2* effects, enables highly sensitive images with respect to motion and diffusion (15). DW-SSFP only requires moderate gradient strength and moderate diffusion gradient duration, and provides better spatial resolution than DW-EPI. Therefore, we aimed to compare the performance of DW-SSFP to DW-EPI in the diagnosis of vertebral compression fractures.
Our data demonstrate that signal intensities measured on DW-SSFP and DW-EPI correlate with each other in both neoplastic and benign osteoporotic fractures. DW-SSFP had comparable specificity to DW-EPI in differentiating neoplastic and benign osteoporotic vertebral fractures. The sensitivity and specificity of DW-SSFP were comparable to those of a prior study (10) which reported a sensitivity and specificity of 100.0% and 93.0%, respectively. The high specificity of the DW-SSWP may be explained by the visualization of the obviously strong signal at the fracture site with distinctive anatomic information, which facilitates more accurate localization of lesion for the image analysis. With the qualitative information, DW-SSFP may be more practical to analyze the fracture lesion than the DW-EPI along with shorter image acquisition time. We expect that DW-SSFP can be used as a fast screening sequence for neoplastic vertebral compression fracture, especially for the patients requiring faster scan due to poor general condition for cooperation or breath holding, or claustrophobia.
In our study, the optimal cut-off value for the DW-SSFP was higher (3.0) than the 1.73 value reported in a previous study (4). The difference could be attributable to differences in MR parameters, such as higher b-values and magnetic moments in 3-T MRI. The postulated optimal cut-off value of 1.3 for ADC was consistent with the results of past studies that demonstrated ADC values for neoplastic fractures ranging from 0.19 to 1.04 and values for benign osteoporotic fractures ranging from 0.32 to 2.21. In another study, lower ADC values were obtained in both neoplastic and benign osteoporotic fractures with cut-offs of 0.19 and 0.30, respectively, possibly due to different sample size and patient inclusion criteria (16). The inverse linear correlations between SI norm and ADC in both neoplastic and benign osteoporotic fractures imply that higher signal intensity on DW-SSFP is associated with lower ADC value on DW-EPI, probably from higher tissue cellularity. The negative correlation coefficients were weak to moderate, partly owing to the small sample size. Our result also shows that the high b-value of 1400 was useful to provide higher resolution and reduce T2 shine-through effects, whereas previous studies utilized b-values smaller than 1000 (478914).
Regardless of background bone marrow signal intensity varying among individuals due to red marrow hyperplasia, focal fat deposition, or other etiologies, neoplastic vertebral fractures restrict diffusion due to increased cellular contents. Meanwhile, diffusion is facilitated in acute osteoporotic vertebral fractures due to reactive fluid and extra-cellular contents, even in the presence of hemorrhagic contents or complex fracture involving pedicle which may mimic neoplastic vertebral fractures (5). Therefore, we assume that there is a low possibility of false-negative or false-positive diagnosis in this study, as fracture lesions were evaluated based on tissue cellularity demonstrated on DW images.
This study has some limitations. First, the sample size was relatively small, and the two patient groups were not matched in terms of age and sex. Further study with larger population and age and sex-matched cohort may better demonstrate the relationship between both sequences. Second, pathologic confirmation for the diagnosis of neoplastic and benign osteoporotic fractures was not performed due to retrospective nature of the study and its invasiveness. However, the confidence of the diagnosis was enhanced by the combination of clinical information, MRI features on DW sequences as well as T1- and T2-weighted sequences, and follow-up imaging studies.
In conclusion, in 3-T MRI, DW-SSFP had comparable sensitivity (90.4% vs. 90.5%) and specificity (95.3% vs. 70.4%) to DW-EPI in differentiating neoplastic vertebral fractures from benign osteoporotic vertebral fractures. In the neoplastic fractures, the median SI norm on DW-SSFP was higher and the median ADC on DW-EPI was lower than the benign osteoporotic fractures (5.24 vs. 1.30 and 0.86 vs. 1.48, respectively). Inverse linear correlations were shown between SI norm and ADC in both neoplastic and benign osteoporotic fractures (r = −0.45 and r = −0.61, respectively). Thus, DW-SSFP may be as useful as DW-EPI in the clinical practice with better resolution for fracture site localization and reduced scanning time.
Figures and Tables
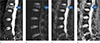 | Fig. 1Benign osteoporotic vertebral fracture in a 57-year-old female who presented with low back pain. Fat-saturated T2-weighted image (T2WI) (a) demonstrates recent vertebral fracture at L1 vertebra with depression of superior end-plate and retropulsion of posterior corner, causing mild narrowing of central canal (arrows in a-d) (b) Diffusion-weighted steady-state free precession (DW-SSFP) demonstrates slightly increased signal intensity compared to the normal bone marrow, with the normalized signal intensity (SI norm) of 1.6. (c, d) Diffusion-weighted echo-planar imaging (DW-EPI) with the b-value of 1400 (c) and ADC of 1.2 (d) show no evidence of diffusion restriction. The ROI measurements are designated as circles. |
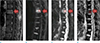 | Fig. 2Neoplastic vertebral fracture of a 60-year-old female with breast cancer who presented with back pain. Fat saturated T2WI (a) demonstrates vertebral fracture with bone marrow-replacing T2 high signal intensity lesion at T8 vertebra (arrows in a-d) (b) DW-SSFP demonstrates markedly increased signal intensity compared to the normal bone marrow, with SI norm of 11.5. (c, d) DW-EPI with the b-values of 1400 (c) and ADC of 0.60 (d) clearly demonstrate diffusion restriction. The ROI measurements are designated as circles. |
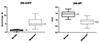 | Fig. 3Box plots of SI norm on DW-SSFP and ADC on DW-EPI in the neoplastic and benign osteoporotic vertebral fracture groups. In DW-SSFP, the neoplastic fracture group has significantly higher SI norm than the benign fracture group (median: 5.24 vs. 1.30, P = 0.032). In DW-EPI, the neoplastic fracture group demonstrates significantly lower ADC than the benign fracture group (median: 0.86 vs. 1.48, P = 0.041). |
 | Fig. 4Correlations between the SI norm of DW-SSFP and ADC of DW-EPI in the neoplastic and benign osteoporotic vertebral fracture groups. Inverse linear correlations are shown between the SI norm and ADC in both neoplastic fractures (r = −0.45) and benign osteoporotic fractures (r = −0.61). |
 | Fig. 5The receiver operating characteristic curves obtained using Youden's J statistics. The optimal cut-off values for diagnosis of neoplastic fracture are SI norm of 3.0 in DW-SSFP with the sensitivity and specificity of 90.4% (95% CI: 81.0–99.0) and 95.3% (95% CI: 90.0–100.0), respectively, and ADC of 1.3 in DW-EPI with the sensitivity and specificity of 90.5% (95% CI: 80.0–100.0) and 70.4% (95% CI: 60.0–80.0), respectively. |
References
1. Kaloostian PE, Yurter A, Zadnik PL, Sciubba DM, Gokaslan ZL. Current paradigms for metastatic spinal disease: an evidence-based review. Ann Surg Oncol. 2014; 21:248–262.
2. An HS, Andreshak TG, Nguyen C, Williams A, Daniels D. Can we distinguish between benign versus malignant compression fractures of the spine by magnetic resonance imaging? Spine (Phila Pa 1976). 1995; 20:1776–1782.
3. Uetani M, Hashmi R, Hayashi K. Malignant and benign compression fractures: differentiation and diagnostic pitfalls on MRI. Clin Radiol. 2004; 59:124–131.
4. Bhugaloo A, Abdullah B, Siow Y, Ng Kh. Diffusion weighted MR imaging in acute vertebral compression fractures: differentiation between malignant and benign causes. Biomed Imaging Interv J. 2006; 2:e12.
5. Jung HS, Jee WH, McCauley TR, Ha KY, Choi KH. Discrimination of metastatic from acute osteoporotic compression spinal fractures with MR imaging. Radiographics. 2003; 23:179–187.
6. Ho CS, Choi WM, Chen CY, Chen WY, Chan WP. Metastasis in vertebra mimicking acute compression fractures in a patient with osteoporosis: MRI findings. Clin Imaging. 2005; 29:64–67.
7. Le Bihan DJ. Differentiation of benign versus pathologic compression fractures with diffusion-weighted MR imaging: a closer step toward the “holy grail” of tissue characterization? Radiology. 1998; 207:305–307.
8. Shih TT, Huang KM, Li YW. Solitary vertebral collapse: distinction between benign and malignant causes using MR patterns. J Magn Reson Imaging. 1999; 9:635–642.
9. Castillo M, Arbelaez A, Smith JK, Fisher LL. Diffusion-weighted MR imaging offers no advantage over routine noncontrast MR imaging in the detection of vertebral metastases. AJNR Am J Neuroradiol. 2000; 21:948–953.
10. Baur A, Huber A, Ertl-Wagner B, et al. Diagnostic value of increased diffusion weighting of a steady-state free precession sequence for differentiating acute benign osteoporotic fractures from pathologic vertebral compression fractures. AJNR Am J Neuroradiol. 2001; 22:366–372.
11. International Osteoporosis Foundation web site. The Asian Audit: Epidemiology, costs and burden of osteoporosis in Asia 2009. Accessed March 18, 2017. http://www.iofbonehealth.org/. Published September 2009.
12. Park SH, Han PK, Choi SH. Physiological and functional magnetic resonance imaging using balanced steady-state free precession. Korean J Radiol. 2015; 16:550–559.
13. Geith T, Schmidt G, Biffar A, et al. Comparison of qualitative and quantitative evaluation of diffusion-weighted MRI and chemical-shift imaging in the differentiation of benign and malignant vertebral body fractures. AJR Am J Roentgenol. 2012; 199:1083–1092.
14. Biffar A, Baur-Melnyk A, Schmidt GP, Reiser MF, Dietrich O. Quantitative analysis of the diffusion-weighted steady-state free precession signal in vertebral bone marrow lesions. Invest Radiol. 2011; 46:601–609.
15. Chavhan GB, Babyn PS, Jankharia BG, Cheng HL, Shroff MM. Steady-state MR imaging sequences: physics, classification, and clinical applications. Radiographics. 2008; 28:1147–1160.
16. Thawait SK, Marcus MA, Morrison WB, Klufas RA, Eng J, Carrino JA. Research synthesis: what is the diagnostic performance of magnetic resonance imaging to discriminate benign from malignant vertebral compression fractures? Systematic review and meta-analysis. Spine (Phila Pa 1976). 2012; 37:E736–E774.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download