Abstract
Purpose
To optimize the timing of scans using cardiac magnetic resonance contrast-enhanced timing robust angiography (CMR-CENTRA) for electroanatomic mapping (EAM) of the right atrium (RA) and left atrium (LA) in patients with atrial fibrillation (AF).
Materials and Methods
Fifty patients with AF (38 men; mean age, 59.6 ± 9.3 years) underwent CMR-CENTRA in preparation for EAM. The CMR-CENTRA data were acquired at five different scan times: 0 seconds, 5 seconds, 10 seconds, 15 seconds, and 20 seconds after an intravenous injection of contrast media. To evaluate the degree of contrast enhancement, right atrial relative contrast (RA-RC) and left atrial relative contrast (LA-RC) on the CMR-CENTRA scans were assessed at each time point. The three-dimensional (3D) reconstruction of the RA and LA for the EAM system was performed using the CMR-CENTRA data.
Results
A CMR-CENTRA at a scan time of 10 seconds showed significantly greater LA-RC (P < 0.05) compared with all other scan times. A CMR-CENTRA at a scan time of 15 seconds showed significantly greater RA-RC (P < 0.05) compared with all other scan times. In the 3D reconstruction of the RA, the success rates of CMR-CENTRA at scan times of 10 seconds and 15 seconds were 18% and 100%, respectively. In the 3D reconstruction of the LA, the success rates of CMR-CENTRA at 10- and 15-second scan times were 100%.
Atrial fibrillation (AF) is a common arrhythmia associated with significant morbidity and mortality when uncontrolled (12). A catheter ablation for the electrical isolation of the AF arrhythmogenic substrate has gained wide acceptance in the management of drug-refractory and symptomatic AF (34). Usually, the AF arrhythmogenic substrates are in various heart structures such as the superior vena cava (SVC), inferior vena cava (IVC), coronary sinus (CS), pulmonary vein (PV), left atrium (LA), left atrial appendage (LAA), and right atrium (RA). Therefore, in the management of AF by a catheter ablation, suitable targets include the LA, LAA, RA and adjacent vessel structures (56).
Accurate knowledge of the cardiac anatomy is essential for the successful application of a catheter ablation for AF. Recently, cardiac magnetic resonance (CMR) imaging with excellent resolution has provided accurate and detailed delineation of the complex cardiac anatomy (789). With technical advancements, a three-dimensional (3D) reconstruction of the heart from CMR image data may be possible according to the catheter-based electrophysiology studies that discuss electroanatomic mapping (EAM) (1011). A 3D reconstruction model of the heart guides catheter movement as it approaches ablation targets and improves the outcomes of catheter ablations in patients with AF (1011). Thus, the 3D reconstruction of the heart via an EAM system requires high-quality cardiac images with excellent spatial resolution and tissue contrast in order to ensure a successful catheter ablation (1011).
Cardiac magnetic resonance contrast-enhanced timing robust angiography (CMR-CENTRA), which is a type of contrast-enhanced magnetic resonance angiography, is characterized by a special form of k-space acquisition that allows for the rapid acquisition of contrast information (12). Thus, it can provide multiphase images containing detailed anatomy and flow dynamics based on the presence of contrast media (12). To the best of our knowledge, however, the CMR-CENTRA protocol for EAM has yet to be established. Therefore, we aimed to determine the optimal scan time of CMR-CENTRA for the 3D reconstruction of the RA and LA as part of the preparation process for EAM in patients with AF.
This retrospective study was approved by the hospital's Institutional Review Board, and all patients provided written informed consent to use their image data and clinical information. The inclusion criteria of this study were: (a) drug-refractory symptomatic AF and (b) available CMR-CENTRA data before an EAM of AF. Based on the hospital information system records, we selected 53 patients with drug-refractory symptomatic AF who underwent CMR-CENTRA in preparation for EAM between March 2015 and May 2015. Exclusion criteria were the presence of either congestive heart failure or congenital cardiac anomaly (e.g., atrial septal defect and ventricular septal defect). In total, 50 patients (38 men; mean age, 59.6 ± 9.3) were retrospectively enrolled into the present study. In all of the patients, the drug-refractory AF included two phenotypes: (a) paroxysmal AF (PAF; patients with one episode of AF that self-terminated within 7 days, n = 38) and (b) persistent AF (PeAF; patients with one episode of AF that lasts more than 7 days, n = 12) (1).
CMR-CENTRA was performed using a 3T MR system (Achieva; Philips Medical Systems, Best, The Netherlands) with a 32-element phased-array cardiac coil. CMR-CENTRA data were acquired in a coronal orientation after the injection of 0.1 mmol/kg of gadolinium contrast (Dotarem; Guerbet, S.A., Villepinte, France). The CMR-CENTRA was completed during one breath-hold without any electrocardiography (ECG) gating. The parameters of the CMR-CENTRA sequence were as follows: (1) parallel imaging using the sensitivity encoding (SENSE) technique with R = 2, acquisition time = 5 s; (2) a total of five phased CMR-CENTRA acquisitions; and (3) a voxel size = 1 mm × 1 mm × 1.2 mm; TR/TE = 3.9 ms/1.1 ms; flip angle = 25°; bandwidth = 1106.2 Hz/pixel. Contrast media was injected at a rate of 2 mL/s via an 18-gauge needle in the left or right cubital vein by an automated power injector (Spectris Solaris EP; Medrad, Indianola, PA, USA); this was followed by an injection of a 40-mL normal saline bolus at the same rate. The CMR-CENTRA scan was initiated at five different scan times: 0 seconds, 5 seconds, 10 seconds, 15 seconds, and 20 seconds after the completion of all injections (Fig. 1). Ultimately, the CMR-CENTRA data consisted of five multiphase cardiac images.
Using a workstation with a commercially available software (Terarecon iNtuition; TeraRecon, Foster City, CA, USA), CMR-CENTRA data were evaluated by two radiologists who had 3 and 10 years of experience in interpreting cardiac imaging data.
With regard to the flow dynamics of the contrast media, parallel imaging of CMR-CENTRA does not allow for the absolute quantitative measure of the signal-to-noise ratio (7). Thus, the degree of contrast enhancement was determined by the relative contrast (RC) of the signal (7). For signal measurement, the regions of interest (ROI) with a minimum area of 100 mm2 were placed in the RA, LA, and chest wall on CMR-CENTRA at each of the five different scan times. The right atrial relative contrast (RA-RC) was calculated using the following formula: RA-RC = (RA signal − chest wall signal) / (RA signal + chest wall signal). The left atrial relative contrast (LA-RC) was calculated using the following formula: LA-RC = (LA signal − chest wall signal) / (LA signal + chest wall signal). The measurements of RA-RC and LA-RC were repeated after an interval of at least 2 weeks.
After the 3D reconstruction of the RA and LA models based on the CMR-CENTRA data (Fig. 2), the image quality of the RA and LA models was evaluated. When the RA model contained no image artifacts and had clear anatomical outlines of the RA, SVC, IVC, and CS, it was considered the ideal RA model. Other RA models were considered imperfect RA models. Similarly, LA models that contained no image artifact and had clear anatomical outlines of the LA, LAA and PVs were considered ideal LA models. Other LA models were considered imperfect models. Then, using all of the CMR-CENTRA examinations performed at identical scan times, the frequency percentages of the ideal RA and LA models were calculated as the success rates for the 3D reconstruction of the RA and LA, respectively.
All continuous data were expressed as mean ± standard deviation (SD). All categorical data were presented as absolute values and percentages. In the measurements of RA-RC and LA-RC, intra-observer reproducibility of the measurement was evaluated by determining the intra-class correlation coefficient (ICC) value (13). An ICC value of greater than or equal to 0.7 was considered statistically reproducible (13). The statistical significance of differences was evaluated using a Student's t-test or analysis of variance (ANOVA) and a pairwise comparison technique (i.e., Tukey's honest significant difference [HSD] test). Statistical analyses were performed using the MedCalc software (version 15.5; MedCalc Software, Ostend, Belgium). A P-value of less than 0.05 was considered statistically significant.
Table 1 summarizes the clinical information of all 50 patients (38 men; mean age, 59.6 ± 9.3 years). When considering the clinical history (e.g., hypertension, diabetes, heart failure, and stroke) (2), the mean CHADS2 score (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, and stroke) was 0.43 ± 0.02.
The mean RA-RC and LA-RC of all of the 250 CMR-CENTRA examinations in the 50 patients were 0.66 ± 0.10 and 0.70 ± 0.13, respectively. Intra-observer reproducibility of the RA-RC and LA-RC measurements was reliable (ICC of RA-RC = 0.81, P = 0.02 and ICC of LA-RC = 0.79, P = 0.01). When comparing the RA-RC values among the five different scan times (Fig. 3), the RA-RC increased until 15 seconds and then peaked. The mean RA-RC at the 15-second scan time was significantly higher compared to that found at the 0-, 5-, 10- and 20-second scan times (all Ps < 0.05). When comparing the LA-RC values among the five different scan times (Fig. 3), the LA-RC increased until 10 seconds and then peaked. The mean LA-RC at the 10-second scan time was significantly higher than that found at the 0-, 5-, 15- and 20-second scan times (all Ps < 0.05).
Out of all of the 250 RA models reconstructed from CMR-CENTRA, there were 88 (35.2%) ideal RA models. The CMR-CENTRA of the ideal RA model showed a significantly higher mean RA-RC (0.77 ± 0.05 vs. 0.61 ± 0.07, P < 0.01) than that found in the imperfect RA model (Fig. 4). The success rates for the 3D reconstruction of the RA by CMR-CENTRA at scan times of 0 seconds, 5 seconds, 10 seconds, 15 seconds, and 20 seconds were 0%, 2%, 18%, 100%, and 22%, respectively (Fig. 5). Out of all of the 250 LA models reconstructed from CMR-CENTRA, there were 115 (46.0%) ideal LA models. The CMR-CENTRA of the ideal LA model showed a significantly higher mean LA-RC (0.82 ± 0.05 vs. 0.60 ± 0.09, P < 0.01) than that found in the imperfect LA model (Fig. 4). The success rates for the 3D reconstruction of the LA by CMR-CENTRA at scan times of 0 seconds, 5 seconds, 10 seconds, 15 seconds, and 20 seconds were 0%, 8%, 100%, 100%, and 22%, respectively (Fig. 5).
This study evaluated the degree of contrast enhancement and image quality on the RA and LA on CMR-CENTRA scans at five different scan times (0 seconds, 5 seconds, 10 seconds, 15 seconds, and 20 seconds after an intravenous injection of contrast media). Review of the LA-RC and RA-RC values as determined by CMR-CENTRA demonstrates that the peak contrast enhancement was observed at 10 seconds and 15 seconds in the LA and RA, respectively. CMR-CENTRA requires appropriate contrast enhancement in the RA and LA in order to perform a successful EAM. In this study, all CMR-CENTRA scans performed 15 seconds after the contrast injection allowed for the successful 3D reconstruction of both the RA and LA in preparation for an EAM for patients with AF.
CMR-CENTRA is a dynamic approach to contrast-enhanced angiography that involves rapid sequential imaging of an anatomic volume during the luminal transit of a contrast bolus (78). CMR-CENTRA involves the use of a T1-weighted gradient echo sequence with a short repetition-time (TR) to allow repeated imaging at a temporal resolution of less than 2 seconds per frame (14). The potential value of CMR-CENTRA in clinical practice is to permit continuous imaging throughout the arterial and venous phases of the contrast enhancement (7814). In the present study, we required a total of 5 seconds to complete a single CMR-CENTRA examination covering the entire heart. Furthermore, a series of five CMR-CENTRA examinations with an interval of 5 seconds can help assess the sequential change of contrast media dynamics throughout the bilateral heart chambers during a period of 20 seconds after the injection of a contrast media.
In CMR-CENTRA, the order of peak contrast enhancement in the heart chambers can depend on the cardiac anatomy. The RA receives the inferior and superior vena cava and the orifice of the coronary sinus (1516). When the contrast media is injected into the cubital vein, it initially enters the RA through the superior vena cava (17). Therefore, the initial combination of opacified and non-opacified blood from the systemic veins interrupts the homogenous contrast enhancement in the RA chamber (1517). In other words, the homogeneous contrast enhancement of the RA can be achieved with the opacified blood drained from both the inferior and superior vena cava. Alternatively, the LA receives blood only from the PV but not from the systemic veins (1819). Without the abnormal pulmonary venous return, the homogeneous contrast enhancement in the LA can be easily achieved by the opacified blood from the PV. Our results also demonstrated that the peak contrast enhancement in the LA occurred before it did in the RA on CMR-CENTRA.
The integration of imaging with electrophysiological studies requires excellent image quality on CMR-CENTRA that focuses on the targeted cardiac structure of the arrhythmogenic substrate (8). The image quality of CMR-CENTRA depends primarily on the degree of contrast enhancement (78). Our results showed that the mean RA-RC was 0.77 ± 0.05 in the acceptable scans of the RA, and the mean LA-RC was 0.82 ± 0.05 in the acceptable scans of the LA. On CMR-CENTRA scans, at the 15-second scan time, the mean RA-RC and LA-RC were 0.79 ± 0.06 and 0.79 ± 0.03, respectively. Although the minimum RC on CMR-CENTRA for a successful EAM of the RA and LA has not been established, we estimate that within 5 seconds after the peak contrast enhancement of the LA at the 10-second scan time, the LA-RC on CMR-CENTRA can allow for the successful EAM of the LA.
Commonly, the electroanatomic mapping of AF has been conducted using cardiac computed tomography (CT) data. When compared with the use of cardiac CT, various techniques of CMR imaging has several advantages for the electroanatomic mapping of AF. First, CMR imaging can prevent the radiation exposure to patients in contrast to a cardiac CT (8). Second, the excellent temporal resolution of CMR imaging can minimize the effect of arrhythmia that is caused by image artifacts. Paradoxically, ECG-gating CMR imaging during an arrhythmic attack may increase the image acquisition time and lead to adverse effects on the image quality (20). Third, CMR imaging allows the comprehensive evaluation of cardiac hemodynamics and tissue characteristics to enhance the understanding of AF (621). As CMR techniques advance, CMR imaging has been widely accepted as the effective pre-procedural imaging tool in the electroanatomic mapping of AF.
This study has several limitations. First, this study was mainly limited by its small sample size. A further study is needed to evaluate patients with congenital cardiac anomalies such as atrial septal defects and abnormal pulmonary venous return. Second, we did not consider additional variables such as cardiac function and rhythm status during CMR-CENTRA in the current study because of the excellent temporal resolution of CMR-CENTRA. Finally, future studies are required to demonstrate the clinical effect of optimized CMR-CENTRA in the process of catheter ablation for patients with AF.
In conclusion, when contrast media is injected into the cubital vein, multiphase images of CMR-CENTRA may show a peak contrast enhancement of the RA after the peak contrast enhancement of the LA. Furthermore, CMR-CENTRA performed 15 seconds after injection of contrast media commonly showed a peak contrast enhancement of the RA and may be appropriate for the successful EAM of the RA and LA chambers in patients with AF.
Figures and Tables
Fig. 1
Sequential CMR-CENTRA images for the visualization of the contrast media passing through the heart. Coronal images (a) of CMR-CENTRA at 0s, 5s, 10s, 15s, and 20s show the dynamics of contrast media in the left atrium (white arrows). In addition, coronal images of CMR-CENTRA (b) at 0s, 5s, 10s, 15s, and 20s show the dynamics of contrast media in the right atrium. The CMR-CENTRA at 15s shows the weak but homogeneous contrast enhancement in the right atrium as well as the superior vena cava. CMR-CENTRA = cardiac magnetic resonance contrast-enhanced timing robust angiography; LA-RC = left atrial relative contrast; RA-RC = right atrial relative contrast
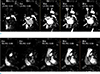
Fig. 2
3D-reconstruction of the right atrium (RA) and left atrium (LA) by using cardiac magnetic resonance contrast-enhanced timing robust angiography (CMR-CENTRA) data acquired at 15s. The 3D-recontstruction of RA and LA from CMR-CENTRA (a, b) provides the ideal RA and LA models (c). Ideal RA model (c) shows the superior vena cava (SVC), inferior vena cava (IVC), coronary sinus (CS), and RA clearly. Ideal LA model (c) shows the pulmonary vein (PV), left atrial appendage (LAA), and LA clearly.

Fig. 3
Comparison of the right atrial relative contrast (RA-RC) (a) and left atrial relative contrast (LA-RC) (b) among the five different scan times of cardiac magnetic resonance contrast-enhanced timing robust angiography (CMR-CENTRA).

Fig. 4
Comparison of the right atrial relative contrast (RA-RC) (a) and left atrial relative contrast (LA-RC) (b) between the ideal and imperfect models of the right atrium (RA) and left atrium (LA).

Fig. 5
The success rates for the 3D reconstruction of the right atrium (a) and left atrium (b) using cardiac magnetic resonance contrast-enhanced timing robust angiography (CMR-CENTRA).

References
1. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114:e257–e354.
2. European Heart Rhythm Association. Heart Rhythm Society. Fuster V, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006; 48:854–906.
3. Haissaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005; 16:1125–1137.
4. Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009; 53:782–789.
5. Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004; 43:2044–2053.
6. Hwang SH, Oh YW, Lee DI, Shim J, Park SW, Kim YH. Relation between left atrial wall composition by late gadolinium enhancement and complex fractionated atrial electrograms in patients with persistent atrial fibrillation: influence of non-fibrotic substrate in the left atrium. Int J Cardiovasc Imaging. 2015; 31:1191–1199.
7. Dabir D, Naehle CP, Clauberg R, Gieseke J, Schild HH, Thomas D. High-resolution motion compensated MRA in patients with congenital heart disease using extracellular contrast agent at 3 Tesla. J Cardiovasc Magn Reson. 2012; 14:75.
8. Schonberger M, Usman A, Galizia M, Popescu A, Collins J, Carr JC. Time-resolved MR venography of the pulmonary veins precatheter-based ablation for atrial fibrillation. J Magn Reson Imaging. 2013; 37:127–137.
9. Yoon YE, Hong YJ, Kim HK, et al. 2014 Korean guidelines for appropriate utilization of cardiovascular magnetic resonance imaging: a joint report of the Korean Society of Cardiology and the Korean Society of Radiology. Korean J Radiol. 2014; 15:659–688.
10. Heist EK, Chevalier J, Holmvang G, et al. Factors affecting error in integration of electroanatomic mapping with CT and MR imaging during catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006; 17:21–27.
11. Bertaglia E, Brandolino G, Zoppo F, Zerbo F, Pascotto P. Integration of three-dimensional left atrial magnetic resonance images into a real-time electroanatomic mapping system: validation of a registration method. Pacing Clin Electrophysiol. 2008; 31:273–282.
12. Jeong HJ, Vakil P, Sheehan JJ, et al. Time-resolved magnetic resonance angiography: evaluation of intrapulmonary circulation parameters in pulmonary arterial hypertension. J Magn Reson Imaging. 2011; 33:225–231.
13. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979; 86:420–428.
14. Giesel FL, Runge V, Kirchin M, et al. Three-dimensional multiphase time-resolved low-dose contrast-enhanced magnetic resonance angiography using TWIST on a 32-channel coil at 3 T: a quantitative and qualitative comparison of a conventional gadolinium chelate with a high-relaxivity agent. J Comput Assist Tomogr. 2010; 34:678–683.
15. Malik SB, Kwan D, Shah AB, Hsu JY. The right atrium: gateway to the heart--anatomic and pathologic imaging findings. Radiographics. 2015; 35:14–31.
16. Callan P, Clark AL. Right heart catheterisation: indications and interpretation. Heart. 2016; 102:147–157.
17. Hwang SH, Oh YW. Assessment of cor triatriatum dexter and giant eustachian valve with cardiac magnetic resonance. Circulation. 2014; 130:1727–1729.
18. Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999; 10:1525–1533.
19. Lacomis JM, Wigginton W, Fuhrman C, Schwartzman D, Armfield DR, Pealer KM. Multi-detector row CT of the left atrium and pulmonary veins before radio-frequency catheter ablation for atrial fibrillation. Radiographics. 2003; 23(Spec No):S35–S48. discussion S48-50.
20. Groarke JD, Waller AH, Vita TS, et al. Feasibility study of electrocardiographic and respiratory gated, gadolinium enhanced magnetic resonance angiography of pulmonary veins and the impact of heart rate and rhythm on study quality. J Cardiovasc Magn Reson. 2014; 16:43.
21. Hwang SH, Roh SY, Shim J, Choi JI, Kim YH, Oh YW. Atrial fibrillation: relationship between left atrial pressure and left atrial appendage emptying determined with velocityencoded cardiac MR imaging. Radiology. 2017; 284:381–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download