Abstract
Purpose
To evaluate the clinical significance of T1 high signal intensity on the globus pallidus as a predictor of severe hepatic encephalopathy in patients with acute-on-chronic liver failure (ACLF), which is a distinct syndrome characterized by multi-organ dysfunction including cerebral failure.
Materials and Methods
From January 2002 to April 2014, we retrospectively reviewed the magnetic resonance imaging (MRI) findings and clinical and magnetic resonance (MR) features of 74 consecutive patients (44 men and 30 women; mean age, 59.5 years) with liver cirrhosis. The chronic liver failure-sequential organ failure assessment score was used to diagnose ACLF. The pallidal index (PI), calculated by dividing the mean signal intensity of the globus pallidus by that of the subcortical frontal white matter were compared according to ACLF. The PI was compared with the Model for End-Stage Liver Disease (MELD) score in predicting the development of ACLF.
Results
Fifteen patients who were diagnosed with ACLF had higher hepatic encephalopathy grades (initial, P = 0.024; follow-up, P = 0.002), MELD scores (P < 0.001), and PI (P = 0.048). In the ACLF group, the mean PI in patients with cerebral failure was significantly higher than that in the patients without cerebral failure (1.33 vs. 1.20, P = 0.039). In patients with ACLF, the area under the curve (AUC) for PI was 0.680 (95% confidence intervals [CI], 0.52–0.85), which was significantly lower than that for the MELD score (AUC, 0.88; 95% CI, 0.77–0.99) (P = 0.04).
Hepatic encephalopathy, a severe neuropsychiatric complication of both acute and chronic hepatic failure, is a major cause of death in patients with liver cirrhosis (1). Depending upon the duration and degree of hepatic dysfunction, hepatic encephalopathy manifests as a spectrum that ranges from minimal disturbances in mental functioning that impact quality of life to coma (2). A recent study revealed that hepatic encephalopathy is associated with higher mortality in patients with acute-on-chronic liver failure (ACLF), which is a new clinical entity with significant morbidity and mortality.
Unlike acute liver failure that occurs in patients without an underlying liver disease, ACLF occurs in patients with previously known or unknown cirrhosis and may rapidly progress to intracranial hypertension and cerebral edema culminating in coma and/or death that is associated with single or multi-organ failure including the liver, kidneys, brain, coagulation, circulation, and lungs (3). Therefore, there is a significant morbidity and mortality in ACLF patients and it is crucial to predict the development of ACLF in patients with liver cirrhosis.
The pallidal high signal intensity (PHSI) on a T1-weighted image (T1-WI) of a brain magnetic resonance imaging (MRI) is a unique finding that is typically observed in more than 75% of the patients who have cirrhosis (456). Since the degree and extension of PHSI seem to correlate with the severity of the liver disease (7), the relative measurement of PHSI could be used as biomarker of brain manganese accumulation (89). Although the disappearance of the PHSI after liver transplantation were confirmed in several reports, there have been reports against a positive correlation of this image with chronic liver insufficiency (10). Therefore, the clinical significance of this abnormality in neuropsychiatric disturbances still remains to be clarified. In the current study, we hypothesized that PHSI can be used as a biomarker for the development of ACLF, which has a high risk of fulminant hepatic failure (FHF) in patients with liver cirrhosis. Therefore, the purpose of this study was to evaluate the clinical significance of PHSI as a predictor of ACLF in patients with liver cirrhosis.
Between January 2002 and April 2014, we retrospectively reviewed the brain magnetic resonance (MR) images and clinical features of 74 consecutive patients who had evidence of liver dysfunction and neuropsychiatric abnormalities including personality change, intellectual impairment, involuntary movement, seizure, and a depressed level of consciousness, as revealed by a clinical diagnosis of hepatic encephalopathy from Kyungpook National University Hospitals. Since this study was retrospective, the Institutional Review Board of our hospital approved this study and waived informed consent.
The study population consisted of 44 men and 30 women that ranged in age from 33 to 84 years (mean age, 59.5 years; median age, 58.0 years). We reviewed clinical data from the medical records, including the Glasgow Coma Scale and encephalopathy status which was determined according to the West-Haven criteria (11). We also assessed the laboratory data on admission, and then calculated the Model for End-Stage Liver Disease (MELD) score to evaluate the hepatic function reserve. The chronic liver failure-sequential organ failure assessment (CLIF-SOFA) was applied to determine organ failure in patients with ACLF as outlined in the CANONIC study (21213). The diagnosis of liver failure was defined as a serum bilirubin level of 12.0 mg/dL or greater. Kidney failure was defined as a serum creatinine level of 2.0 mg/dL or greater or the need for renal replacement therapy. Cerebral failure was defined by grade III or IV hepatic encephalopathy as per the West-Haven classification (14). Coagulation failure included an international normalized ratio (INR) of 2.5 or greater and/or a platelet count of 20,000/cc or less. Circulatory failure was defined by a need for the use of vasopressors like dopamine, dobutamine, or terlipressin at any dose. Respiratory failure was defined by a PaO2 to FiO2 ratio of 200 or less or a SpO2 to FiO2 ratio of 214 or less.
The brain MR imaging was performed with one 1.5T (Signa Excite, GE Healthcare, WI, USA) and two 3T MR imaging units (Signa Excite or Signa HDxt, GE, USA). Axial unenhanced T1-weighted and T2-weighted images were obtained with variable settings. The T1-weighted spin-echo images were acquired with the following parameters: TR 550–630, TE 11–14, flip angle 60–70, section thickness 6 mm, inter-slice gap 0.7–2 mm, matrix size 352 × 192–1024 × 192, FOV 174 × 200–220 × 220, numbers of signal acquired 1–2. The T2-weighted spin-echo images were acquired with the following parameters: TR 3588–4400, TE 99–125, flip angle 90 or 180, section thickness 6 mm, interslice gap 0.7–2 mm, matrix size 512 × 224, FOV 180 × 200.
The signal intensity of the globus pallidus and frontal white matter on the T1-weighted images were measured by one neuroradiologist (M.H.H.) and one resident (D.H.L.) independently. For a quantitative analysis of the signal intensity of the globus pallidus, two operator-defined region-of-interests (ROIs) were placed in the globus pallidus and the ipsilateral subcortical frontal white matter. The pallidal index (PI) was calculated by dividing the mean signal intensity of the globus pallidus by that of the subcortical frontal white matter (Fig. 1).
We compared the clinical and MRI findings of the two different groups (ACLF and non-ACLF) using two-sampled t-tests and chi-squared tests. Because of the relatively small number of patients in the ACLF group, the Fisher's exact test was used when the expected cell frequency was less than 5. To assess the inter-rater reliability of the signal intensity measurement, the intra-class correlation coefficients (ICC, [3, 1]) with 95% confidence intervals (CI) were calculated. The Currier criteria for evaluating ICC values were applied as very reliable (0.80–1.0), moderately reliable (0.60–0.79), and questioned reliable (< 0.60) (15).
A receiver operating characteristic (ROC) analysis was performed to determine the area under the ROC curve (AUC) for assessing the performance of the PI in predicting ACLF. The statistical analysis was performed by using SPSS software, version 20 (IBM, Armonk, NY, USA), and MedCalc software, version 17.8.5 (MedCalc Software, Ostend, Belgium). Statistical significance was set at a P-value of less than 0.05.
The demographic and clinical characteristics of patients with and without ACLF is provided in Table 1. The 15 patients with ACLF included 8 men and 7 women with a mean age of 56.6 ± 11 years (range, 33–84 years). The 59 patients without ACLF included 32 men and 27 women with a mean age of 60.3 ± 10 years (range, 36–80 years). The cause of cirrhosis was not related to the development of ACLF. Initially, the cerebral failure rate was lower in the ACLF group, but at follow-up, it was higher than the rates of the non-ACLF group (P = 0.024, P = 0.002). The except serum sodium level (P = 0.002), blood urea nitrogen (BUN; P = 0.014), total bilirubin (P = 0.013), and INR (P = 0.042) values in the laboratory data were statistically higher in the ACLF group. The MELD score (P < 0.001) was also higher than in the ACLF group.
MRIs were obtained from 54 patients using 1.5T machines and from 20 patents via 3T MR machines. Although the signal intensity of the globus pallidus (871.83 vs. 634.79) and the white matter of the frontal lobe (758.95 vs. 514.50) were higher from the 3T machines, there was no statistical difference in the PI (1.18 ± 0.10 vs. 1.21 ± 0.10, P = 1.99). The scatter diagram displays the change in the PI due to ACLF (Fig. 2). In this diagram, the PI is the regression slope, which is the ratio of the pallidal signal intensity to the subcortical frontal signal intensity. The effect of ACLF on the PI changes in a linear regression analysis was statistically significant (unstandardized coefficients 36.64, 95% confidence interval 1.98–71.34, P = 0.039). The inter-rater ICC (2, k) values of the signal intensity measurements were 0.996 for the globus pallidus and 0.992 for the white matter of the right frontal lobe, which signifies a high inter-reliability.
In particular, the PI elevations in patients with ACLF were associated with cerebral failure (initial hepatic encephalopathy grade ≥ 3). The mean PI of patients with cerebral failure was higher than that of patients without cerebral failure. In the ACLF group, the mean PI of patients with cerebral failure was significantly higher than that of the patients without cerebral failure (1.33 vs. 1.20, P = 0.039) (Fig. 3).
In a ROC curve analysis that compared the predictive accuracy of the PI with the MELD score, the AUC values for the occurrence of ACLF in the patients with cirrhosis were 0.878 for the MELD score (95% confidence interval, 0.765–0.990) and 0.680 for the PI (95% confidence interval, 0.515–0.846). The AUC values of the MELD score were significantly higher than that of the PI (P = 0.040) (Fig. 4).
Manganese is a cofactor for key enzymes such as astrocytic glutamine synthetase, pyruvate carboxylase, and mitochondrial superoxide dismutase, which is responsible for shortening the T1 relaxation time and increasing signal intensities in brain MRIs (8). Because the signal intensity of MRI is not an absolute physiologic value such as the Hounsfield unit in CT, but instead a relative value that is influenced by MR parameters, the degree of the increased signal intensity due to manganese accumulation was measured by a relative value such as the PI, which is defined as the ratio of the signal intensity in the globus pallidus to the subcortical frontal white matter in axial T1-WI planes (16). In the current study, the signal intensities of the globus pallidus were correlated positively with those of the white matter of the frontal lobe. However, the linear correlation exhibited a higher slope in cases with ACLF, which requires meticulous consideration for the clinical application of the PI. Until now, the correlation between the PI and clinical index has revealed the severity of the portal systemic shunt such as whole blood manganese, Child/Pugh score and total bilirubin level (17). However, none of the trials to prove the clinical significance of PI resulted in failure as an index for the development of FHF. Our hypothesis was that the high signal intensity on the globus pallidus correlated with the biomarker related to the development of ACLF, which is a step toward FHF. ACLF has been recognized as a distinct disease entity encompassing an acute deterioration of liver function in patients with chronic liver disease (18).
The mortality probability of patients with ACLF was much higher than that of patients without ACLF (19). Although there are no universally accepted diagnostic criteria for ACLF, several representative consensus definitions have been commonly used except for the CLIF-SOFA. One definition was proposed by the Asian Pacific Association for the Study of the Liver (APASL) which was recently revised in 2014 by the ACLF Research Consortium (18) and the other by the European Association for the Study of the Liver-Chronic Liver Failure Consortium (13). According to the latter, ACLF is a recently recognized syndrome characterized by acute decompensation of cirrhosis and organ or system failure, and renal and cerebral failure are essential for the diagnosis of ACLF (220). In the current study, we used the CLIF-SOFA criteria which focus on multiple organ failures rather than the liver-specific APASL criteria for the definition of ACLF and prognostication even in Asian patients with ACLF. In the CLIF-SOFA system, cerebral failure was defined as grade III or IV hepatic encephalopathy per the West-Haven criteria (14). Hepatic encephalopathy in hospitalized cirrhotic patients is associated with a high mortality rate and its presence adds further to the mortality of patients with ACLF (2).
ACLF has a high risk for the development of FHF. In the current study, the comparison of the PI in patients with hepatic encephalopathy according to ACLF demonstrated that the mean PI in patients with cerebral failure was significantly higher than in patients without cerebral failure in the ACLF group. Therefore, especially in patients with ACLF, a relatively high PI could indicate a relatively high probability for the development of severe encephalopathy which cause clinicians more concern in intensively preventing hepatic encephalopathy. Of course, to determine the critical cut-off value of the PI as a useful predictor of severe encephalopathy in patients with ACLF, further randomized controlled studies are needed. In the current study, the MELD was more useful to predict the development of ACLF than was the PI on brain MRIs. The MELD is a reliable measure of mortality risk in patients with end-stage liver disease. It is used as a disease severity index to help prioritize allocation of organs for transplants. Therefore, the PI could be a limited index in the clinical field.
This study has several limitations. First, the most commonly enrolled underlying disease was HBV-related hepatitis. Whereas the predominant underlying precipitating factors, as a hepatic encephalopathy, is a viral hepatitis occurring in Asia, the predominant factor in western countries is drug-induced liver damage (21). Second, we did not compare the MR features of chronic liver disease without hepatic encephalopathy as a control population. Since a brain MRI is not a mandatory examination in patients with chronic liver disease without hepatic encephalopathy, sufficient brain MR images were not obtained as a control group. Therefore, in order to clarify our hypothesis for this disease entity, prospective studies with a large population is recommended. Third, the included MR images of our patients were from two different magnets. We attempted to normalize the score by dividing by the frontal white matter signal intensity. However, the effect of T1 shortening can be different on the manganese deposited area compared to the non-manganese deposited areas. Finally, since our results were not reinforced by pathological correlations, a general consensus based on intensive clinical reviews should have been followed. The PHSI is similar to those described in patients without chronic liver disease who received enteral nutrition with high concentrations of manganese (22), and miners and metal workers exposed to manganese (1923).
In summary, the current study demonstrated that the PI is significantly related to the development of ACLF and the mean PI in patients with cerebral failure is significantly higher than that of the patients without cerebral failure in the ACLF group. Until now, many approaches of the PI in the clinical field has been attempted; however, this is the first report to apply ACLF as a new disease entity of chronic liver disease to our knowledge. In conclusion, the PI can be an ancillary biomarker for predicting the development of ACLF and severe hepatic encephalopathy.
Figures and Tables
Fig. 1
ROI measurement for the PI. Standard ROIs placed in the globus pallidus on a non-enhanced T1-weighted image displays a high signal intensity globus pallidus. PI = pallidal index; ROI = region of interest
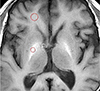
Fig. 2
Linear correlation between the signal intensity of the frontal white matter and globus pallidus, according to acute-on-chronic liver failure. ACLF = acute-on-chronic liver failure; SI frontal = T1 signal intensity on subcortical white matter; SI pallidus = T1 signal intensity on globus pallidus

Fig. 3
Comparison of the pallidal index in patients with hepatic encephalopathy according to ACLF. ACLF = acute-on-chronic liver failure; HE = hepatic encephalopathy

Fig. 4
Comparison of the MELD and PI to predict ACLF on a ROC curve. ACLF = acute-on-chronic liver failure; MELD = Model for End-Stage Liver Disease; PI = pallidal index; ROC = receiver operating characteristic

Table 1
Characteristics of Patients with or without ACLF

Variables are expressed as mean ± standard deviation or number (%).
†P value < 0.05: statistically significant
*Viral: seropositive of hepatitis B antibody or hepatitis C antigen
**Non-viral: alcohol, autoimmune hepatitis, primary and secondary biliary cirrhosis, and unknown causes
ACLF = acute-on-chronic liver failure; GCS = glasgow coma scale; HE = hepatic encephalopathy; INR = international normalized ratio; K = potassium; MELD = model for end-stage liver disease; Na = sodium
References
1. Sherlock S. Hepatic encephalopathy. Br J Hosp Med. 1977; 17:144–146. 151–154. 159
2. Romero-Gomez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015; 62:437–447.
3. Wright G, Sharifi Y, Jover-Cobos M, Jalan R. The brain in acute on chronic liver failure. Metab Brain Dis. 2014; 29:965–973.
4. Inoue E, Hori S, Narumi Y, et al. Portal-systemic encephalopathy: presence of basal ganglia lesions with high signal intensity on MR images. Radiology. 1991; 179:551–555.
5. Pujol A, Pujol J, Graus F, et al. Hyperintense globus pallidus on T1-weighted MRI in cirrhotic patients is associated with severity of liver failure. Neurology. 1993; 43:65–69.
6. Zeneroli ML, Cioni G, Crisi G, Vezzelli C, Ventura E. Globus pallidus alterations and brain atrophy in liver cirrhosis patients with encephalopathy: an MR imaging study. Magn Reson Imaging. 1991; 9:295–302.
7. Burkhard PR, Delavelle J, Du Pasquier R, Spahr L. Chronic parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch Neurol. 2003; 60:521–528.
8. Li SJ, Jiang L, Fu X, et al. Pallidal index as biomarker of manganese brain accumulation and associated with manganese levels in blood: a meta-analysis. PLoS One. 2014; 9:e93900.
9. Chang Y, Woo ST, Kim Y, et al. Pallidal index measured with three-dimensional T1-weighted gradient echo sequence is a good predictor of manganese exposure in welders. J Magn Reson Imaging. 2010; 31:1020–1026.
10. Rovira A, Alonso J, Cordoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol. 2008; 29:1612–1621.
11. Volk ML, Marrero JA. Advances in critical care hepatology. Minerva Anestesiol. 2006; 72:269–281.
12. Delis SG, Bakoyiannis A, Biliatis I, Athanassiou K, Tassopoulos N, Dervenis C. Model for end-stage liver disease (MELD) score, as a prognostic factor for postoperative morbidity and mortality in cirrhotic patients, undergoing hepatectomy for hepatocellular carcinoma. HPB (Oxford). 2009; 11:351–335.
13. Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144:1426–1437. 1437.e1–1437.e9.
14. Blei AT, Cordoba J, Practice Parameters. Hepatic encephalopathy. Am J Gastroenterol. 2001; 96:1968–1976.
15. Elements of research in physical therapy. Baltimore: Lippincott Williams and Wilkins;1990.
16. Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet. 1995; 346:270–274.
17. Spahr L, Butterworth RF, Fontaine S, et al. Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology. 1996; 24:1116–1120.
18. Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009; 3:269–282.
19. Shin YC, Kim E, Cheong HK, et al. High signal intensity on magnetic resonance imaging as a predictor of neurobehavioral performance of workers exposed to manganese. Neurotoxicology. 2007; 28:257–262.
20. Arroyo V, Moreau R, Jalan R, Gines P, EASL-CLIF Consotrium. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015; 62:S131–S143.
21. Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995; 21:240–252.
22. Herrero Hernandez E, Valentini MC, Discalzi G. T1-weighted hyperintensity in basal ganglia at brain magnetic resonance imaging: are different pathologies sharing a common mechanism. Neurotoxicology. 2002; 23:669–674.
23. Criswell SR, Perlmutter JS, Huang JL, et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med. 2012; 69:437–443.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download