Abstract
Dermoid cysts are benign congenital tumors composed of keratinizing squamous epithelium and dermal derivatives. They account for less than 1% of all intracranial tumors and are rarely exhibited at the base of the skull. To the best of our knowledge, only one case report has presented computed tomography and conventional T1-weighted magnetic resonance (MR) findings that revealed an infratemporal dermoid cyst. In the present study, we report an unusual case of a dermoid cyst in the right infratemporal fossa, which was incidentally detected by MR imaging with the Dixon technique. This article also highlights the importance of meticulous radiological review and the usefulness of the Dixon technique in everyday clinical practice.
Skull base dermoid cysts are rare tumors surrounded by keratinizing squamous epithelial cells (1). Because of their congenital origin, these tumors typically arise between infancy and young adulthood (2). They are considered likely to arise from a defective neural tube closure, which occurs between the third and fifth weeks of gestation (2). Most head and neck dermoid cysts occur in intradural midline locations, such as the posterior fossa, suprasellar, parasellar and frontonasal regions (12). Of these, skull base dermoid cysts are extremely rare and a total of 11 cases have been reported in literature, including a solitary case of infratemporal location (3). Here, we present an unusual case of a dermoid cyst in the right infratemporal fossa, which was incidentally detected by magnetic resonance imaging (MRI) with the Dixon technique. We also present a relevant literature review of this disease and a summary of the Dixon technique for detecting fat-containing lesions. To the best of our knowledge, this is the first report of a skull base dermoid cyst diagnosed using the Dixon MRI technique.
A 24-year-old woman visited our hospital with the complaint of recurrent seizures occurring at irregular intervals over the past 10 years. The seizures lasted a few minutes each, with reported symptoms of altered consciousness, and were brought under control with anticonvulsants. The patient had no history of any other neurological or medical disorder, or recent trauma. The findings of a physical examination and laboratory tests revealed no specific abnormalities, including external wounds. No neurological deficits were noticed upon initial neurological examination, and the electroencephalogram and laboratory findings were normal. Therefore, a brain MRI was performed to identify a possible seizure focus. Imaging was performed using a 3T system (Signa Architect; GE Healthcare, Milwaukee, WI, USA) with a 48-channel head coil. We routinely used the Dixon technique for acquiring T2-weighted images to increase the detectability and conspicuity of lesions by robust fat suppression, with following scan parameters: TR, 3860 ms; TE, 100 ms; flip angle, 111°; FOV, 210 × 190 mm; matrix, 320 × 320; section thickness, 5 mm; NEX, 1.0; bandwidth, ± 25 kHz; acquisition time, 2 minutes, 23 seconds. The imaging findings revealed no visible seizure focus. However, there was an incidental finding of a well-circumscribed mass in the right infratemporal fossa, near the foramen ovale, measuring about 2.8 × 1.8 × 2.3 cm in size. On T1-weighted images, the mass-like lesion exhibited an intensely homogenous hyperintensity (Fig. 1a-d). The lesion also exhibited a bright signal intensity with minimal internal inhomogeneity on in-phase T2-weighted images (Fig. 1e). On water-only T2-weighted images acquired using the Dixon technique (Fig. 1f), and a fat-suppressed fluid attenuation inversion recovery image (Fig. 1g), the lesion exhibited a completely uniform suppression of the signal intensity, suggesting the presence of a fat component. There were several small isointense intracystic nodules in the inferior portion. A thin, hypointense rim was noted at the periphery of the lesion on all sequences, which was considered to represent a capsule and the dural layer (Fig. 1a-e). There was no contrast enhancement within the lesion or at the periphery (Fig. 1h). The lesion also exhibited a focal extension to the posterolateral portion near the foramen ovale, without definite involvement. A slight displacement of the trigeminal nerve was also noted. Although microsurgical resection was recommended, the patient wanted regular imaging follow-up without surgical resection.
Dermoid cysts, probably originating from ectodermal remnants, are situated quite close to midline structures and are usually observed during childhood (4). The etiology of these tumors involves primitive ectodermal cells and mesenchymal elements from closing neuroectodermal folds, during weeks 3-5 of gestation. Another etiology is traumatic implantation of skin elements (1). Dermoid cysts are mainly composed of keratinizing squamous epithelium, but also consist of smooth muscle cells and hair follicles, as well as apocrine and sebaceous glands. They may occur anywhere around the skull, including intracranial spaces and the skull vault. Intracranial dermoid cysts most commonly occur in the posterior fossa, around the cerebellar vermis and the fourth ventricle. They occur less frequently in the supraparasellar, pineal, and parapontine regions (4). Dermoid cysts in the head and neck regions have been reported to occur specifically in the fontanels, cavernous sinus, petrous apex, Eustachian tube, periorbital or perinasal soft tissue, and scalp. They tend to originate from skull sutures located near the skin and have a direct connection with the dermal sinus (5). Of these, skull base dermoid cysts are extremely rare, with only 11 cases having been reported in literature, including only one case of infratemporal location (3). To the best of our knowledge, this is the first report of a skull base dermoid cyst occurring in the vicinity of the foramen ovale, which was diagnosed using the Dixon technique.
Patients are commonly asymptomatic or have mild symptoms, such as a headache. However, if dermoid cysts are ruptured by chance or trauma, the intracystic contents are spread into intracranial spaces, which can then lead to chemical meningitis, chronic granulated arachnoiditis, aqueductal stenosis, ventriculitis, or seizures (26).
Dermoid cysts are often confidently diagnosed on the basis of radiological findings. They are typically presented as well-circumscribed, fat-containing masses without contrast enhancement. On computed tomography (CT) images, these cysts characteristically present as markedly hypodense masses with various degrees of inhomogeneity because of variations in the proportions of fatty and non-fatty intracystic components (7). For imaging the diagnosis of dermoid cysts, MRI is demonstrated to be superior to CT, as it provides high signal intensity of the lesion on T1-weighted images and variable signal intensity on T2-weighted images. The signal intensity of MR images can be significantly influenced by fat content, calcification, hair follicles, epithelial debris, sebum, and water content within dermoid cysts (78). Absence of perilesional edema and the presence of well-defined margins with characteristic signal intensity on MR images are helpful in differentiating dermoid cysts from other benign, space-occupying lesions, such as cystic neoplasms, arachnoid cysts and epidermoid cysts.
In the present case, the patient did not undergo brain CT. However, the lesion exhibited a strong hyperintensity on both T1- and T2-weighed images. Therefore, the list of differential diagnoses had a broad range, including hemorrhagic, high proteinaceous, and fatty masses. Fortunately, we made a confident diagnosis on the basis of MRI findings with a single scan, because we had routinely used the fluid attenuation inversion recovery image with fat suppression and the Dixon technique to obtain T2-weighted images. In the present case, the lesion exhibited a uniform dark signal intensity, which suggested it to be a fat-containing lesion based on the fat suppression sequences. Although this case represents a singular experience of clinical use of the Dixon technique, routine use of this technique may be helpful in the process of brain imaging. The technique is valuable because eliminates the need to acquire additional scans for the evaluation of incidentally-detected, intracranial fat-containing lesions, or head and neck lesions below the skull base. It also tends to improve the detectability and conspicuity of lesions in the calvaria and scalp. In addition, it can enhance the sharpness of the overall image quality due to its robust suppression of background fat. However, further study will be required to validate the aforementioned advantages of the Dixon technique in brain MRIs. The Dixon technique, as described by Dixon in 1984, is a chemical shift-based fat-water separation method for imaging. This technique helps to acquire either two different images or two images with two different echo times in each image, in order to decompose the fat signal from the water signal in the same voxel. Because the water and fat spins precess at different frequencies, their magnetization vectors rotate with respect to each other between excitation and acquisition, becoming alternatively “in-phase” (i.e., pointing in the same direction) and “out-of-phase” (i.e., pointing in opposite directions). Acquiring both in-phase and opposed-phase images simultaneously allows the images to be combined mathematically in two ways which result in a total of 4 sequences, as follows: in-phase = (water + fat), opposed-phase = (water - fat), fat-only = in-phase - opposed phase = (water + fat) - (water - fat) and water-only = in-phase + opposed phase = (water + fat) + (water - fat). The water-only image can be used as a fat-suppressed image, and the fat-only image can be used for quantification in certain clinical situations. Therefore, the Dixon technique offers enhanced and signal-to-noise-ratio-efficient fat suppression without the need for additional fat suppression images, as demonstrated in the present case. In addition, because of its insensitivity to B0 and B1 heterogeneity, this technique can be useful for obtaining images in anatomical areas of high susceptibility (910).
For dermoid cysts, complete resection is the only effective treatment modality to prevent recurrence or a potential malignant transformation to squamous cell carcinoma. However, because of the fibrous adhesion of dermoid cysts to adjacent neurovascular structures, complete surgical resection is not always feasible.
Here, we reported a rare case of an incidentally-detected skull base dermoid cyst in the right infratemporal fossa in a patient experiencing recurrent seizures. Through this case report, we hope to highlight the importance of meticulous radiological review for dermoid cysts occurring in blind spots (such as the base of the skull), as well as the usefulness of the Dixon technique in everyday clinical practice.
Figures and Tables
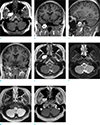 | Fig. 1A 24-year-old woman presented with a complaint of recurrent seizures over the past 10 years. On T1-weighted images (a-d), a well-circumscribed, strongly hyperintense mass with a thin isointense capsule was visible in the right infratemporal fossa. Several small isointense intracystic nodules, visible in the inferior portion of the lesion (arrowheads, b and c), were considered to be an indicator of non-fatty components. The ipsilateral trigeminal nerve in the foramen ovale was clearly delineated, with a mild posterolateral displacement (arrows, a and d). On images acquired using the Dixon technique, the lesion exhibited a bright signal intensity on in-phase, T2-weighted images (e); however, on water-only T2-weighted images, the lesion exhibited uniform hypointensity due to robust fat suppression (f). On fat-suppressed fluid attenuation inversion recovery images (g), the lesion exhibited a homogenous hypointensity as well. The lesion did not exhibit definite enhancement in the internal portion or at the periphery (h). |
Acknowledgments
The authors would like to thank ‘Elsevier Language Editing Service’ for the English language review and editing; http://webshop.elsevier.com/languageservices/languageediting/
References
1. Guidetti B, Gagliardi FM. Epidermoid and dermoid cysts. Clinical evaluation and late surgical results. J Neurosurg. 1977; 47:12–18.
2. Caldarelli M, Massimi L, Kondageski C, Di Rocco C. Intracranial midline dermoid and epidermoid cysts in children. J Neurosurg. 2004; 100:473–480.
3. Watanabe K, Filomena CA, Nonaka Y, et al. Extradural dermoid cyst of the anterior infratemporal fossa. Case report. J Neurol Surg Rep. 2015; 76:e195–e199.
4. Arseni C, Danaila L, Constantinescu AI, Carp N, Decu P. Cerebral dermoid tumours. Neurochirurgia (Stuttg). 1976; 19:104–114.
5. North KN, Antony JH, Johnston IH. Dermoid of cavernous sinus resulting in isolated oculomotor nerve palsy. Pediatr Neurol. 1993; 9:221–223.
6. Oursin C, Wetzel SG, Lyrer P, Bachli H, Stock KW. Ruptured intracranial dermoid cyst. J Neurosurg Sci. 1999; 43:217–220. discussion 220-211.
7. Wilms G, Casselman J, Demaerel P, Plets C, De Haene I, Baert AL. CT and MRI of ruptured intracranial dermoids. Neuroradiology. 1991; 33:149–151.
8. Rubin G, Scienza R, Pasqualin A, Rosta L, DaPian R. Craniocerebral epidermoids and dermoids. A review of 44 cases. Acta Neurochir (Wien). 1989; 97:1–16.
9. Del Grande F, Santini F, Herzka DA, et al. Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics. 2014; 34:217–233.
10. Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984; 153:189–194.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download