Abstract
The perfusion change in acute symptomatic hypoglycemic encephalopathy (ASHE) is not well known. We present the perfusion-weighted imaging of a patient with ASHE. The area of diffusion-weighted imaging abnormalities and adjacent normal-appearing white matter showed increased cerebral blood volume and flow, and shortening of time-to-peak.
The diagnosis of acute symptomatic hypoglycemic encephalopathy (ASHE) is usually evident, and diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map can show early cortical or subcortical abnormalities (1). However, the perfusion change in ASHE is not well known. We herein present the clinical and MRI findings of a patient with ASHE. We will describe the change of perfusion-weighted MRI (PWI) parameter maps, and we will discuss the mechanism and clinical implication of the perfusion change in ASHE.
An 85-year-old woman was transferred to our emergency room due to a stuporous mentality. She received knee surgery at the orthopedic hospital 3 days before admission to our hospital. Stuporous mentality and aphasia developed 2 hours before admission. She has taken medication for diabetes mellitus and hypertension since 10 years before. After knee surgery, her oral intake was poor for three days. In our hospital, she showed global aphasia and left-sided weakness (arm grade 1, leg grade 2). Her blood glucose measured 32 mg/dL. She showed gradual improvement of consciousness after infusion of 50% dextrose via intravenously. MRI including DWI and PWI was performed 3 hours after the onset of symptoms under the suspicion of hypoglycemic encephalopathy or acute stroke. DWI revealed diffusion restriction along the right corona radiata and the more cephalic corticospinal tract (CST). Average ADC value of the lesions was 0.465 × 10-3 mm2/sec. PWI revealed increased perfusion in the DWI abnormalities and adjacent normal-appearing white matter (NAWM) compared to the normal contralateral WM. Region-of-interest analysis was done using Perfusion Mismatch Analyzer (Acute Stroke Imaging Standardization Group, Japan). The DWI abnormalities had increased relative cerebral blood volume and flow (rCBV, rCBF) and shortening of time-to-peak (TTP); rCBV 1.49, rCBF 1.64, TTP 1.2 sec. The adjacent NAWM showed range of increased perfusion; rCBV 1.23-1.53, rCBF 1.33-2.08, TTP shortening 1.1-1.4 sec (Fig. 1). Her hemiparesis resolved completely, and mental state was improved to an alert state on the 2nd day of admission. The DWI abnormalities and perfusion change resolved on follow-up MRI at 1 week. There were no neurological sequelae at the time of discharge.
DWI of ASHE has been well known (1), however, perfusion MRI was rarely described. Before using perfusion MRI, a few studies using perfusion SPECT has mentioned transient perfusion change during insulin-induced hypoglycemia in human subjects (2). According to the literature, CBF compared with basal state increased diffusely in the cerebral hemisphere 21-38% during hypoglycemia, and perfusion change was persistent throughout 15-60 minutes after the reversal of hypoglycemia. Cordonnier et al. (3) reported perfusion MRI in a case of ASHE, and they described 20% increase of rCBV in the DWI lesion of the internal capsule compared to the contralateral WM. However, they did not present a relevant PWI figure. In our study, increased rCBV, rCBF, and shortening of TTP were present in the DWI abnormalities and adjacent NAWM. The area of increased perfusion was larger than the area of DWI abnormality. Although the mechanism of the increased perfusion in ASHE was not clearly defined, hyperperfusion may be a physiological compensatory process to the decreased energy source of the symptomatic area. Our case showed the range of hyperperfusion in the DWI lesion and the surrounding NAWM. This heterogeneity of hemodynamic response to hypoglycemia may be ascribed from different local energy demand, different concentration of neurotransmitters such as glutamate or vascular structural characteristics (pial arterioles versus perforators). In the previous literature, the patients with ASHE had favorable clinical outcome when the DWI abnormalities confined to the focal WM area of CST like as our case (1456). Although this is a single-case experience, localized hyperperfusion in ASHE may mean preservation of compensatory function of CBF control, and it may be associated with good prognosis. The increased perfusion in the DWI abnormality has been described also in the acute phase of mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) (78). ASHE or MELAS can manifest focal neurological deficit like as acute ischemic stroke, and hyperperfusion in the area of DWI abnormality. This finding may be helpful for differential diagnosis between these conditions and acute ischemic stroke.
In conclusion, DSC-MRI revealed hyperperfusion in a case of ASHE. Hyperperfusion was present not only in the DWI abnormalities but also in the adjacent NAWM.
Figures and Tables
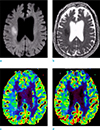 | Fig. 1Diffusion-weighted imaging (DWI) (a) and apparent diffusion coefficient map (b) revealed diffusion restriction along the right corona radiata of the corticospinal tract. Relative cerebral blood volume (c) and relative cerebral blood flow (d) showed increased perfusion in the area of DWI abnormalities and adjacent normal-appearing white matter. Hyperperfusion was predominant in the DWI abnormalities and the deep white matter posterior to the DWI lesion (double arrows), and the white matter near to the cortex (arrows) showed mildly increased perfusion. |
References
1. Yong AW, Morris Z, Shuler K, Smith C, Wardlaw J. Acute symptomatic hypoglycaemia mimicking ischaemic stroke on imaging: a systemic review. BMC Neurol. 2012; 12:139.
2. Tallroth G, Ryding E, Agardh CD. Regional cerebral blood flow in normal man during insulin-induced hypoglycemia and in the recovery period following glucose infusion. Metabolism. 1992; 41:717–721.
3. Cordonnier C, Oppenheim C, Lamy C, Meder JF, Mas JL. Serial diffusion and perfusion-weighted MR in transient hypoglycemia. Neurology. 2005; 65:175.
4. Bottcher J, Kunze A, Kurrat C, et al. Localized reversible reduction of apparent diffusion coefficient in transient hypoglycemia-induced hemiparesis. Stroke. 2005; 36:e20–e22.
5. Lo L, Tan AC, Umapathi T, Lim CC. Diffusion-weighted MR imaging in early diagnosis and prognosis of hypoglycemia. AJNR Am J Neuroradiol. 2006; 27:1222–1224.
6. Aoki T, Sato T, Hasegawa K, Ishizaki R, Saiki M. Reversible hyperintensity lesion on diffusion-weighted MRI in hypoglycemic coma. Neurology. 2004; 63:392–393.
7. Kim JH, Lim MK, Jeon TY, et al. Diffusion and perfusion characteristics of MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode) in thirteen patients. Korean J Radiol. 2011; 12:15–24.
8. Li R, Xiao HF, Lyu JH, JJ Wang D, Ma L, Lou X. Differential diagnosis of mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) and ischemic stroke using 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging. 2017; 45:199–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download