초록
Purpose
To investigate the exchange and redistribution of hyperpolarized13C metabolites between different pools by temporally analyzing the relative fraction of dual T2∗ components of hyperpolarized 13C metabolites.
Materials and Methods
A dual exponential decay analysis of T2∗ is performed for [1-13C] pyruvate and [1-13C] lactate using nonspatially resolved dynamic13C MR spectroscopy from mice brains with tumors (n = 3) and without (n = 4) tumors. The values of shorter and longer T2∗ components are explored when fitted from averaged spectrum and temporal variations of their fractions.
Results
The T2∗ values were not significantly different between the tumor and control groups, but the fraction of longer T2∗ [1-13C] lactate components was more than 10% in the tumor group over that of the controls (P < 0.1). The fraction of shorter T2∗ components of [1-13C] pyruvate showed an increasing tendency while that of the [1-13C] lactate was decreasing over time. The slopes of the changing fraction were steeper for the tumor group than the controls, especially for lactate (P < 0.01). In both pyruvate and lactate, the fraction of the shorter T2∗ component was always greater than the longer T2∗ component over time.
Conclusion
The exchange and redistribution of pyruvate and lactate between different pools was investigated by dual component analysis of the free induction decay signal from hyperpolarized 13C experiments. Tumor and control groups showed differences in their fractions rather than the values of longer and shorter T2∗ components. Fraction changing dynamics may provide an aspect for extravasation and membrane transport of pyruvate and lactate, and will be useful to determine the appropriate time window for acquisition of hyperpolarized 13C images.
REFERENCES
1. Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003; 100:10158–10163.
2. Kurhanewicz J, Vigneron DB, Brindle K, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 2011; 13:81–97.

3. Day SE, Kettunen MI, Gallagher FA, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007; 13:1382–1387.

5. Kettunen MI, Kennedy BW, Hu DE, Brindle KM. Spin echo measurements of the extravasation and tumor cell uptake of hyperpolarized [1-(13) C]lactate and [1-(13) C]pyruvate. Magn Reson Med. 2013; 70:1200–1209.
6. Romijn JA, Chinkes DL, Schwarz JM, Wolfe RR. Lactate-pyruvate interconversion in blood: implications for in vivo tracer studies. Am J Physiol. 1994; 266:E334–340.

7. Yen YF, Le Roux PR, Bok R, et al. Apparent T2 of 13C-labeled metabolites in vivo. In. Proceedings of the 16th Annual Meeting of ISMRM. Toronto: Canada;2008. p. 1747.
8. Kettunen MI, Hu DE, Witney TH, et al. Magnetization transfer measurements of exchange between hyperpolarized [1-13C]pyruvate and [1-13C]lactate in a murine lymphoma. Magn Reson Med. 2010; 63:872–880.

9. Carr HY, Purcell EM. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev. 1954; 94:630–638.

10. Whittall KP, Mackay AL. Quantitative interpretation of NMR relaxation data. J Magn Reson. 1969; 84:134–152.

Fig. 1.
Cellular spaces (metabolic pools) associated with exchange and redistribution of pyruvate and lactate.
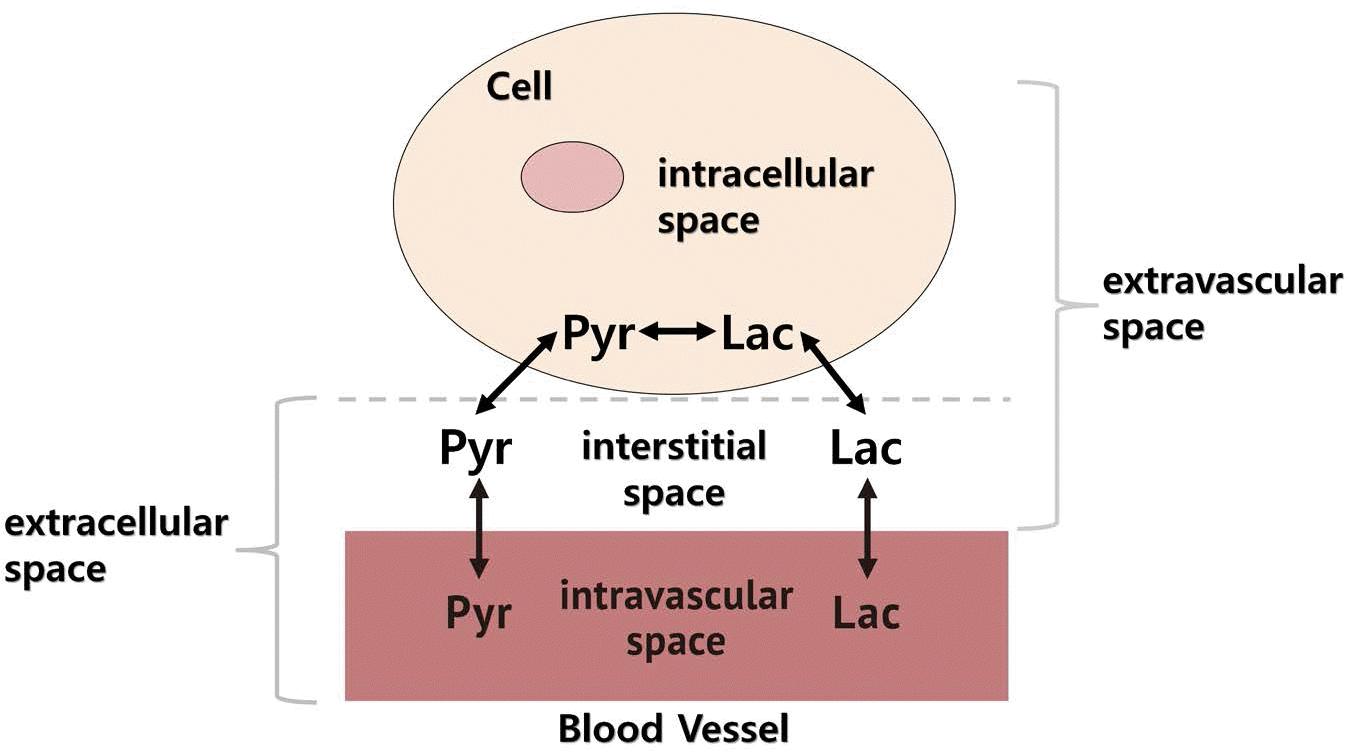
Fig. 2.
1H T2 weighted anatomical images on (a) axial and (b) coronal slices. The yellow box indicates the slice of excitation. (c) Time domain apodization with 100 ms decaying exponential, (d) 300 Hz Gaussian window in spectral domain, (e) extracted decay curves of lactate (enlarged) and pyruvate with its fitting line.
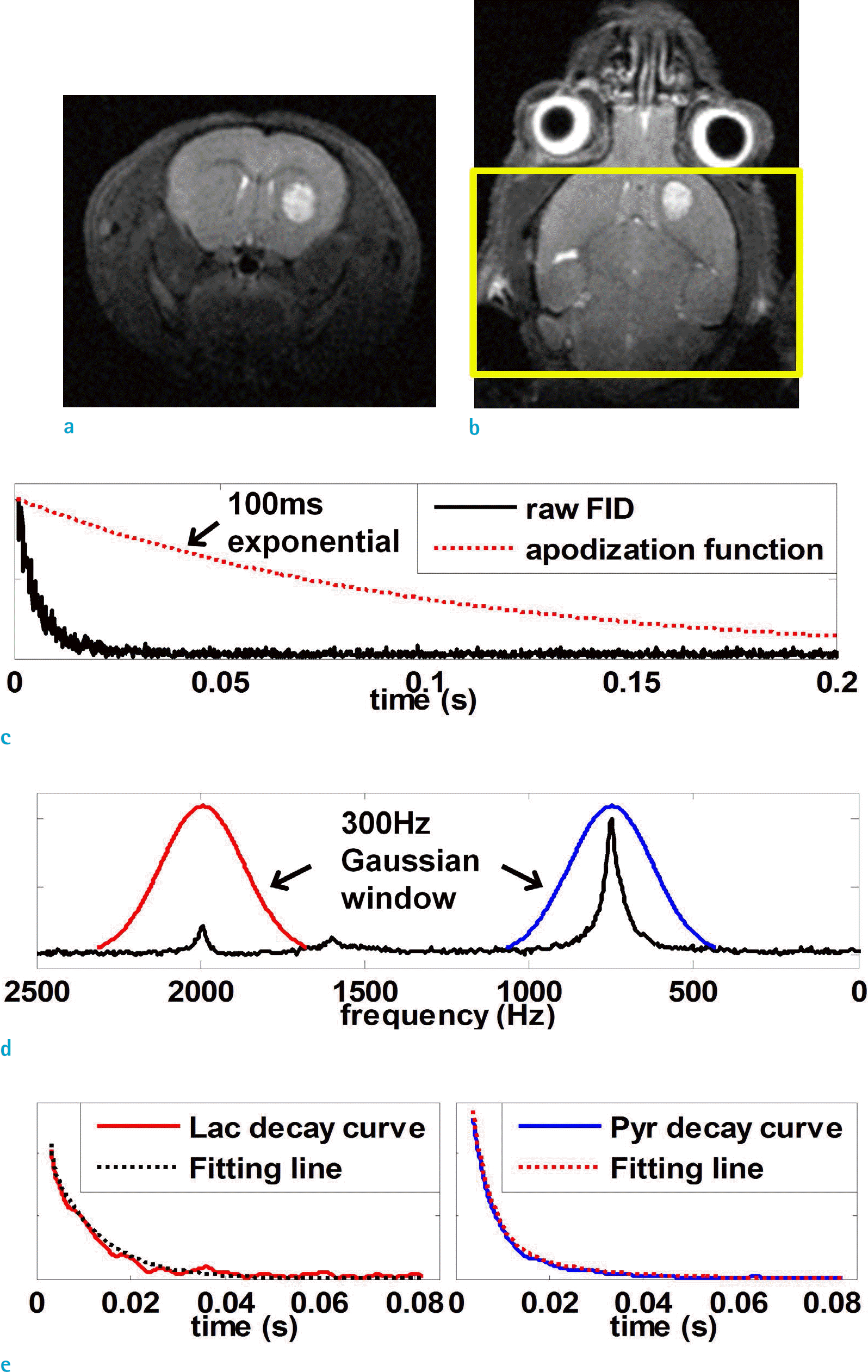
Fig. 3.
(a) T2∗ values and (b) their relative fraction of [1-13C] pyruvate and lactate of averaged spectrum.
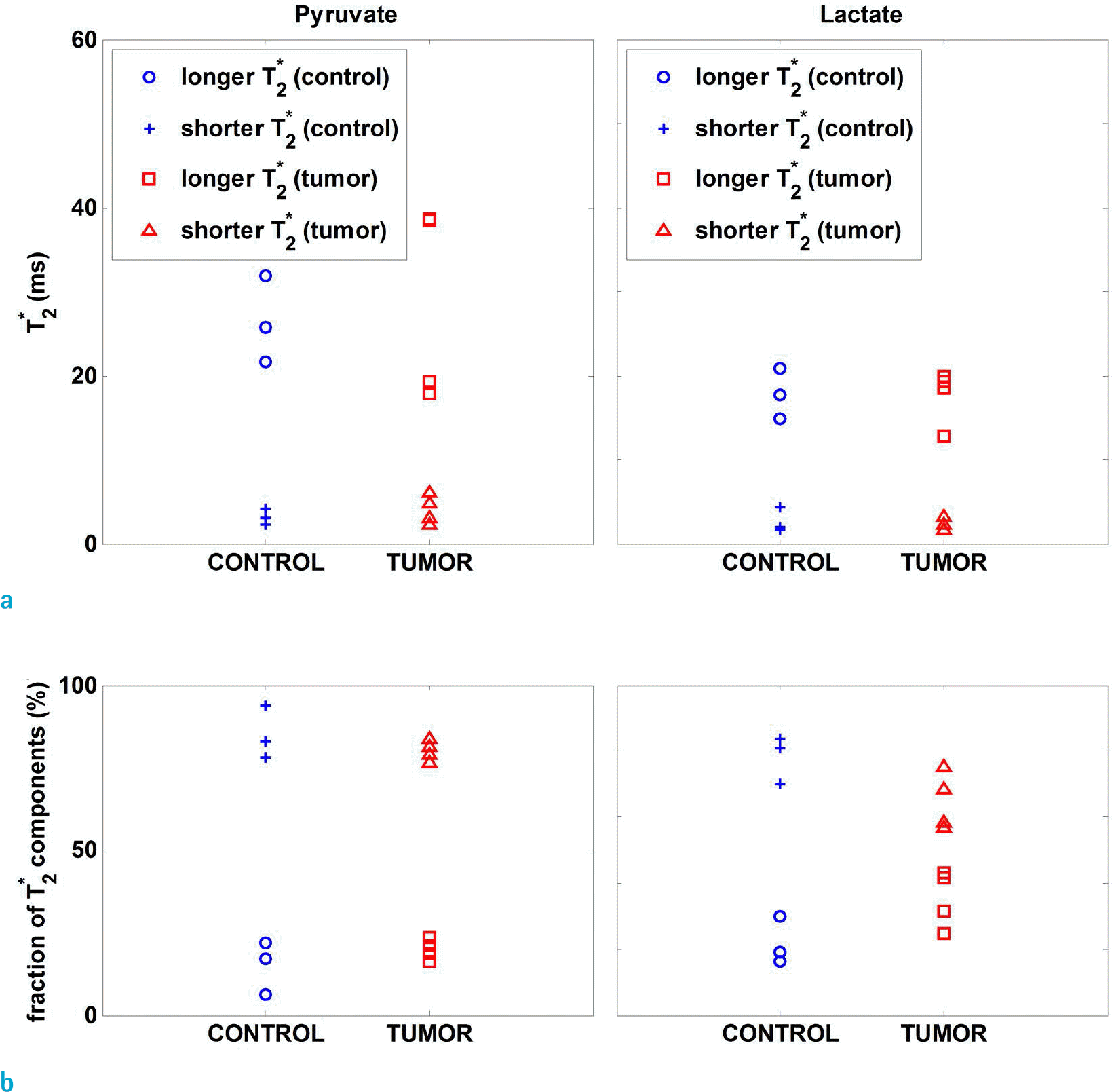
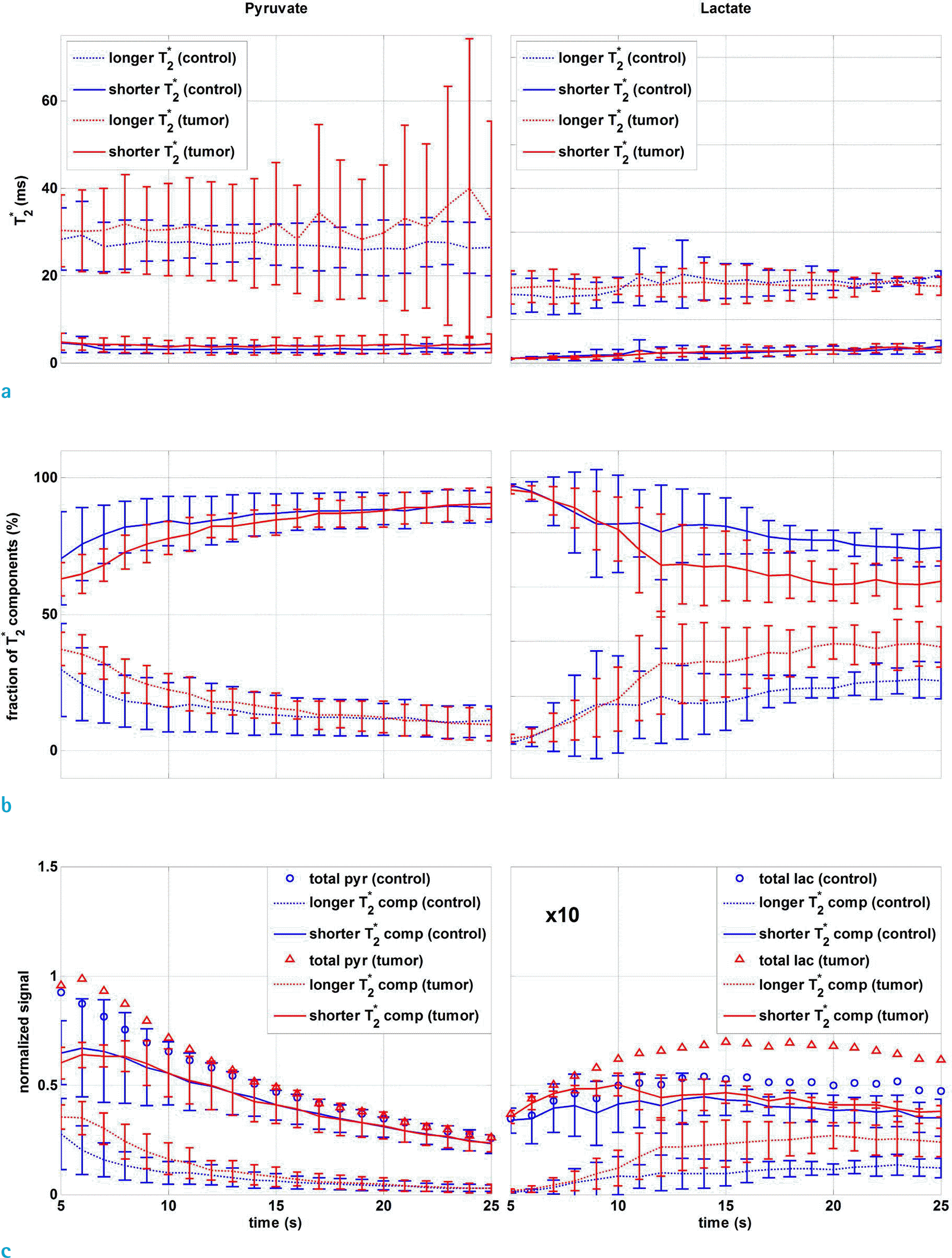
Fig. 4.
Temporal change of (a) T2∗ values [1-13C] pyruvate and lactate and (b) their relative fractions for the tumor (red) and control (blue) groups. (c) Dynamic signals of [1-13C] pyruvate and lactate, showing the total, shorter and longer T2∗ components of the tumor and control groups. Each dynamic curve was normalized by maximum pyruvate signal intensity. Mean and standard deviation of inter-subject signal are indicated. Injection started at t = 0.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download