Abstract
Purpose
Fluid-attenuated inversion recovery (FLAIR) imaging can be obtained faster with shorter repletion time (TR), but it gets noisier. We hypothesized that shorter-TR FLAIR obtained at 3 tesla (3T) with a 32-channel coil may be comparable to conventional FLAIR. The aim of this study was to compare the diagnostic value between conventional FLAIR (TR = 9000 ms, FLAIR9000) and shorter-TR FLAIR (TR = 6000 ms, FLAIR6000) at 3T in terms of diffusion-weighted imaging-FLAIR mismatch.
Materials and Methods
We recruited 184 patients with acute ischemic stroke (28 patients < 4.5 hours) who had undergone 5-mm diffusion-weighted imaging (DWI) and two successive 5-mm FLAIR images (no gap; in-plane resolution, 0.9 × 0.9 mm) at 3T with a 32-channel coil. The acquisition times for FLAIR9000 and FLAIR6000 were 108 seconds (generalized autocalibrating partially parallel acquisitions [GRAPPA] = 2) and 60 seconds (GRAPPA = 3), respectively. Two radiologists independently assessed the paired imaging sets (DWI-FLAIR9000 and DWI-FLAIR6000) for the presence of matched hyperintense lesions on each FLAIR imaging. The signal intensity ratios (area of DWI lesion to contralateral normal-appearing region) on both FLAIR imaging sets were compared.
Results
DWI-FLAIR9000 mismatch was present in 39 of 184 (21.2%) patients, which was perfectly the same on FLAIR6000. Three of 145 patients (2%) with DWI-matched lesions on FLAIR9000 had discrepancy on FLAIR6000, showing no significant difference (P > 0.05). Interobserver agreement was excellent for both DWI-FLAIR9000 and DWI-FLAIR6000 (k = 0.904 and 0.883, respectively). Between the two FLAIR imaging sets, there was no significant difference of signal intensity ratio (mean, standard deviation; 1.25 ± 0.20; 1.24 ± 0.20, respectively) (P > 0.05).
Acute ischemic stroke is a major life-threatening disease that needs treatment as soon as possible. Intravenous thrombolysis (IVT) is of proven and substantial benefit for patients who present within 4.5 hours. Thus, it is very important to know the exact stroke onset time (123) because IVT can only be applied to patients who present within the specific time window for treatment. Unfortunately, however, many patients with acute ischemic stroke present with unknown onset time, which limits the number of eligible patients (456). Although CT is the most widely available and faster imaging modality, magnetic resonance imaging (MRI) may play a critical role in the evaluation of these patients in that fluid-attenuated inversion recovery (FLAIR) imaging may help to determine the age of infarct by diffusion-weighted imaging (DWI)-FLAIR mismatch concept (789). By taking advantage of such a simple, but meaningful imaging strategy, a few clinical trials are underway (1011). In such circumstances, DWI and FLAIR are the mainstay of imaging studies, and should be obtained as fast as possible because time is brain for patient with acute stroke. Thus, there is a need for further improvement in acquisition speed.
A few studies suggested that echo-planar FLAIR and echo-planar gradient-recalled echo (GRE) accelerated by parallel imaging can be a good alternative to conventional imaging to reduce acquisition time (12131415). However, echo-planar GRE FLAIR images are susceptible to artifact, particularly in the regions of air-bone interface, and have relatively lower signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) compared to conventional FLAIR imaging (Fig. 1). FLAIR imaging can be obtained faster with shorter repetition time (TR), but it is noisier than that with conventional TR (9000-10000 ms). With the advent of a 32-channel coil, we can obtain MR images with higher SNR than those with 20- or less channel coil. We hypothesized that FLAIR with shorter TR may be comparable to conventional FLAIR by taking advantage of a 32-channel coil at 3T.
The aim of this study was to compare the diagnostic value between FLAIR with TR of 9000 ms (conventional FLAIR) and FLAIR with TR of 6000 ms (shorter-repetition-time FLAIR) at 3T in patients with acute ischemic stroke.
This study was approved by our Institutional Review Board, and informed consent of subjects was given by patients or their guardians.
We enrolled patients who presented with acute ischemic stroke and who had initially undergone 5-mm DWI and two successive 5-mm FLAIR images at 3T with a 32-channel coil between September 2014 and March 2015. This imaging protocol was only effective during the study period. We excluded patients who did not have restricted diffusion on DWI. Also, patients who had severe artifacts (n = 6) and too small hyperintense lesion (< 10 mm2) on images (n = 21) were excluded. A total of 184 patients (81 female; mean age, 69.3 years) met our inclusion criteria and they had acute ischemic stroke (known [n = 121; 28 patients < 4.5 hours, 93 patients ≥ 4.5 hours] and unknown symptom onset times [n = 63]). In patients with known onset time, the ranges of the onset time to MR imaging was from 20 minutes to 344 hours (median, 15.9 hours; interquartile ranges, 4.8-46.4 hours). The median and interquartile ranges of the baseline NIHSS scores were 4.0 and 2-14, respectively.
MR imaging was performed at a 3.0T MR imager (Skyra; Siemens, Erlangen, Germany) with a 32-channel head coil. DWI was performed with an echo-planar spin-echo sequence with repetition time (TR) of 5900 ms, echo time (TE) of 80 ms, a matrix number of 192 x 192, two b values of 0 and 1000 s/mm2, field of view (FOV) of 220 mm, and no gap. FLAIR with TR of 9000 ms (FLAIR9000) images were obtained with the following parameters: TR/TE/inversion time (TI) = 9000/89/2500; matrix, 256 x 256; field of view (FOV), 250 mm; number of signals acquired, 1; in-plane resolution, 0.9 × 0.9 mm; no gap; a reduction factor of two; acquisition time, 108 seconds. FLAIR with TR of 6000 ms (FLAIR6000) images were acquired with TR/TE/TI = 6000/89/2027; a reduction factor of three; acquisition time, 60 seconds; all other parameters were identical to those for FLAIR9000.
The paired imaging sets (DWI-FLAIR9000 and DWIFLAIR6000) were analyzed independently by two radiologists (one with 20 years and the other with 4 years of experience in radiology) at separate sessions two weeks apart. The presence or absence of matched hyperintense lesions (> 10 mm2 on DWI) on each FLAIR imaging was evaluated. When we encountered multiple infarcted lesions, we measured the largest one among the hyperintense lesions on DWI. Then, the signal intensity ratios between the area of DWI lesion and contralateral normal-appearing region on both FLAIR imaging sets were compared by drawing regions of interest by the second reviewer (16).
Inter-observer agreement was assessed using Cohen's kappa statistics between the two reviewers. Discrepancy was resolved by consensus. The McNemar test was used to determine whether the two FLAIR images (FLAIR 9000 vs. FLAIR6000) were significantly different from each other. The signal intensity ratios (SIR) between FLAIR9000 and FLAIR6000 were compared by paired t-test. Statistical significance was set at P < 0.05, and statistical analyses were performed using SPSS (version 21; IBM, Armonk, NY, USA).
The median volume of infarcted area on DWI in all patients (n = 184) was 107.1 mm2 (interquartile range, 48.7-370.1 mm2). Interobserver agreement was excellent for both DWI-FLAIR9000 and DWI-FLAIR6000 (Cohen's kappa = 0.904 and 0.883, respectively). After consensus review, DWI-FLAIR9000 mismatch was present in 39 of 184 (21.2%) patients, which was similarly observed on FLAIR6000 (Fig. 2). The median volume of hyperintense lesions on DWI in this groups was 96.0 mm2 (interquartile range, 47.3-349.9 mm2) and the time from presentation to MRI ranged from 1 to 12 hours (median, 3.3 hr; interquartile range, 2.5-6.9 hr). FLAIR6000 showed DWI-matched hyperintense lesions in 142 out of 184 (77.2%) patients, which were identically observed on FLAIR9000. The median volume of DWI lesions in this groups was 114.9 mm2 (interquartile range, 50.6-370.9 mm2) and the time from presentation to MRI ranged from 0.3 to 344.4 hours (median, 26.7 hr; interquartile range, 9.6-54.0 hr).
The mean SIRs on FLAIR9000 and FLAIR6000 for DWI-positive lesions were 1.25 ± 0.20 and 1.24 ± 0.20, respectively. The mean SIRs on FLAIR 9000 and FLAIR 6000 was 1.15 ± 0.19 and 1.14 ± 0.18 for patients with time of onset to MRI of < 4.5 hours (n = 28), 1.27 ± 0.21 and 1.27 ± 0.20 for > 4.5 hours (n = 93) and 1.26 ± 0.17 and 1.25 ± 0.19 (n = 63) for unknown onset, respectively (Table 1). Between the two FLAIR imaging sets, there was no significant difference of SIR (P > 0.05), irrespective of onset time. Between the patients who obtained MRI within 4.5 hours and beyond 4.5 hours after the symptom onset, the mean SIRs of both FLAIR9000 and FLAIR6000 were statistically higher in the latter (1.15 ± 0.19 vs. 1.27 ± 0.21, P = 0.006 for FLAIR9000; 1.14 ± 0.18 vs. 1.27 ± 0.20, P = 0.003 for FLAIR6000), respectively.
In terms of DWI-FLAIR match, there was no statistically significant difference between FLAIR9000 and FLAIR6000 (P > 0.05), although there was discrepancy between the two FLAIR imaging sets in three patients in which FLAIR9000 only showed matched lesions in the left basal ganglia, pons, and left temporal lobe (onset time to MRI, 53/76 hours/unknown, respectively) (Fig. 3). In these three patients, the mean SIR on FLAIR6000 was slightly lower than on FLAIR9000 (1.11 ± 0.09 and 1.19 ± 0.15, respectively), but it was difficult to conduct statistical assessment due to too small number. When compared to patients with time of onset to MRI > 4.5 hours, these three patients exhibited relatively lower SIR (1.26 ± 0.20 vs. 1.11 ± 0.09 on FLAIR6000). They had relatively smaller volume of DWI hyperintense lesions (pons, 28.87 mm2; left temporal, 15.50 mm2; left basal ganglia, 10.80 mm2).
All of 8 patients with DWI lesion volumes less than 30 mm2 (19.95 ± 4.68 mm2) exhibited mismatch between DWI and both FLAIR imaging sets. They showed SIR of 1.19 ± 0.14 (FLAIR9000) and 1.22 ± 0.13 (FLAIR6000). Aside from the two patients with unknown onset time, 6 of them presented relatively earlier (3.2, 6.9, 8.6, 10.4, 10.8, and 12.1 hours, respectively [8.87 ± 3.24]) than the two patients who had known onset time and showed discrepancy between the two FLAIR imaging set.
In this study, we compared the diagnostic value between conventional FLAIR (TR = 9000 ms, FLAIR9000) and shorter-TR FLAIR (TR = 6000 ms, FLAIR6000) at 3T in terms of DWI-FLAIR mismatch, and found no significant difference between conventional FLAIR and shorter-TR FLAIR in determining DWI-FLAIR mismatch by both visual and semiquantitative assessment. From our results, we may obtain FLAIR imaging faster than conventional one without compromise of benefit of DWI-FLAIR mismatch concept. We were able to obtain FLAIR imaging with a FLAIR6000 protocol in 60 seconds, which is an approximately 2-fold reduction in scan time compared to conventional FLAIR (108 seconds).
It has been estimated that for every minute during which ischemic stroke is left untreated, approximately 1.9 million neurons are lost (17). Thus, it is very important to obtain diagnostic imaging as soon as possible in acute ischemic stroke. CT is preferred to this end since it is much faster than MRI. However, it has lower sensitivity in the detection of infarct core than MRI, and also has the downside of imposing radiation on patients. In such circumstances, faster MRI techniques are desirable to reduce acquisition time. In previous studies, echo-planar imaging (EPI) in combination with parallel imaging was utilized to reduce the scan time for patients with acute ischemic stroke (1215), in which they were able to obtain whole-brain EPI FLAIR within a minute. However, the drawbacks of such a technique are susceptibility artifact and lower SNR (Fig. 1). Instead of EPI FLAIR, we obtained turbo spin-echo FLAIR with shorter TR to reduce acquisition time. Thanks to a 32-channel coil, we were able to obtain FLAIR imaging with similar diagnostic performance by maintaining similar SIR when compared to conventional FLAIR imaging. Moreover, our shorter-TR FLAIR imaging does not suffer from susceptibility artifact unlike EPI FLAIR imaging, which may be beneficial for assessment of basal frontal lobe and temporal lobe near the skull base.
In our study, there were three patients who showed the matched DWI lesions with those on FLAIR9000, but not with FLAIR6000. These lesions are relatively small (28.87 mm2 in pons, 15.5 mm2 in left temporal lobe, and 10.8 mm2 in left basal ganglia). They showed relatively lower SIR on FLAIR6000 than FLAIR9000 (1.11 ± 0.09 and 1.19 ± 0.15, respectively) and had smaller DWI lesions. It is difficult to explain why the SIRs on FLAIR6000 in these 3 patients were slightly lower than those on FLAIR9000. Two of them presented relatively late than 6 patients with similar DWI lesion volume (< 30 mm2), and might have fogging phenomenon in small lesions. Also, FLAIR6000 may suffer from slightly higher noise. Thus, these 3 factors (late onset time, smaller volume, and higher noise level) independently or together might affect lower SIRs of FLAIR6000 patients presented later. However, we admit it is just speculation and needs further evaluation to determine the exact cause of such discrepancy. This discrepancy may limit the utility of FLAIR6000. In this study, however, the majority of patients had relatively large infarcts (median, 107.10 mm2; interquartile range, 48.7-370.1 mm2), and showed statistically similar diagnostic performance between FLAIR9000 and FLAIR6000. Given that the original concept of DWI-FLAIR mismatch is for determining further intervention such as intravenous fibrinolysis or mechanical thrombectomy, such patients with smaller DWI lesions that showed mismatch between FLAIR9000 and FLAIR6000 may not be a big concern.
Although we were able to obtain faster FLAIR imaging without significant compromise of diagnostic performance by taking advantage of a 32-channel coil in our study, such hardware is not widely available, limiting universal utilization. Rather, it would be better to apply a relatively new fast imaging technique such as simultaneous multislice (SMS) imaging (18). As the SMS for FLAIR was not available when we conducted our study, which may be the case in most of research institutes, we were unable to apply this in our study. We may be able to further reduce the scan time with a higher signal-to-noise ratio than that of FLAIR6000 by applying such a fancy imaging technique.
We note several limitations to our study. First, we reviewed imaging data in a retrospective manner without assessment of outcomes in a single center. Although our study goals did not include the clinical outcomes, it may have added further information for consideration. Second, this study involved a relatively small number of subjects. Third, our protocol requires 3T MRI with a 32-channel coil, which limits a wide use of our faster imaging strategy. As mentioned above, however, an approach utilizing the SMS techniques could be a good alternative.
In conclusion, for the determination of mismatch or match between DWI and FLAIR imaging, there is no significant difference between FLAIR9000 and FLAIR6000 at 3T with a 32-channel coil. Therefore, FLAIR with shorter TR can be applied to patients with acute stroke to reduce acquisition time.
Figures and Tables
Fig. 1
Echo-planar GRE FLAIR versus conventional FLAIR. Echo-planar GRE FLAIR is relatively noisier than conventional FLAIR, and has artifacts in the basal frontal and temporal lobes on both sides (arrows). DWI = diffusion-weighted imaging; EPI = echo-planar imaging; FLAIR = fluid-attenuated inversion recovery; GRE = gradient-recalled echo
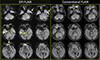
Fig. 2
DWI-FLAIR mismatch and match: agreement between FLAIR9000 and FLAIR6000. DWI and FLAIR images were obtained 64 minutes after symptom onset (upper row). DWI lesions (arrows) are not conspicuously visualized on either conventional FLAIR (FLAIR9000) or FLAIR with shorter TR (FLAIR6000). DWI and FLAIR images were acquired 520 minutes after symptom onset (bottom row). DWI lesions are similarly matched on both conventional FLAIR (FLAIR9000) and FLAIR with shorter TR (FLAIR6000). DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery; TR = repetition time
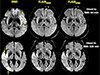
Fig. 3
DWI-FLAIR match: disagreement between FLAIR9000 and FLAIR6000. This DWI lesion in the pons (arrow) is visualized in the same area on conventional FLAIR (FLAIR9000) (arrowhead), but not conspicuously seen on FLAIR with shorter TR (FLAIR6000). DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery; TR = repetition time
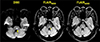
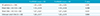
Table 1
Signal Intensity Ratios between FLAIR9000 and FLAIR6000
References
1. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004; 363:768–774.
2. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009; 40:2945–2948.
3. Balami JS, Hadley G, Sutherland BA, Karbalai H, Buchan AM. The exact science of stroke thrombolysis and the quiet art of patient selection. Brain. 2013; 136:3528–3553.
4. European Stroke Organisation (ESO) Executive Committee. ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008; 25:457–507.
5. Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007; 115:e478–e534.
6. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001; 56:1015–1020.
7. Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009; 65:724–732.
8. Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PREFLAIR): a multicentre observational study. Lancet Neurol. 2011; 10:978–986.
9. Petkova M, Rodrigo S, Lamy C, et al. MR imaging helps predict time from symptom onset in patients with acute stroke: implications for patients with unknown onset time. Radiology. 2010; 257:782–792.
10. Koga M, Toyoda K, Kimura K, et al. THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0.6 mg/kg (THAWS) Trial. Int J Stroke. 2014; 9:1117–1124.
11. Thomalla G, Fiebach JB, Ostergaard L, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP). Int J Stroke. 2014; 9:829–836.
12. Nael K, Khan R, Choudhary G, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries. Stroke. 2014; 45:1985–1991.
13. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002; 47:1202–1210.
14. Wiesinger F, Van de Moortele PF, Adriany G, De Zanche N, Ugurbil K, Pruessmann KP. Potential and feasibility of parallel MRI at high field. NMR Biomed. 2006; 19:368–378.
15. Meshksar A, Villablanca JP, Khan R, Carmody R, Coull B, Nael K. Role of EPI-FLAIR in patients with acute stroke: a comparative analysis with FLAIR. AJNR Am J Neuroradiol. 2014; 35:878–883.
16. Galinovic I, Puig J, Neeb L, et al. Visual and region of interest-based inter-rater agreement in the assessment of the diffusion-weighted imaging- fluid-attenuated inversion recovery mismatch. Stroke. 2014; 45:1170–1172.
17. Saver JL. Time is brain--quantified. Stroke. 2006; 37:263–266.
18. Gagoski BA, Bilgic B, Eichner C, et al. RARE/turbo spin echo imaging with simultaneous multislice wave-CAIPI. Magn Reson Med. 2015; 73:929–938.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download