Abstract
We report a case of perivalvular abscess in a 66-year-old man with infective endocarditis, diagnosed by late gadolinium-enhanced (LGE) cardiovascular magnetic resonance (CMR) imaging. No clinical features suspicious of infective endocarditis were noted, however, transthoracic echocardiography revealed non-specific echogenic focal wall thickening at mitral-aortic intervalvular fibrosa. Perivalvular abscess in the aortic valve was demonstrated as focal wall thickening between the anterior mitral leaflet and the non-coronary cusp of the aortic valve with peripheral enhancement and central low signal intensity on LGE CMR imaging. Other features suggestive of infective endocarditis, such as neither vegetation nor valvular perforation were present. The perivalvular abscess did not grow after intensive intravenous antibiotics therapy, and the patient was discharged without surgical treatment. CMR with LGE provided an early accurate diagnosis of perivalvular abscess. The diagnosis of perivalvular abscess using LGE CMR imaging was not previously reported in Korea.
The diagnosis of infective endocarditis (IE) is combination of clinical features and evidence of endocardial involvement. Echocardiography is the key component in the diagnosis and determining the treatment in patients with IE. Major criteria in diagnosis of IE includes endocardial involvement including oscillating intracardiac mass or vegetation, an annular abscess, prosthetic valve partial dehiscence, and new valvular regurgitation (1). Transesophageal echocardiography (TEE) is known to be more sensitive when compared to transthoracic echocardiography (TTE) in diagnosis of IE (2). However, even TEE may show false negative result in perivalvular abscess, especially in the early course of the disease, and only demonstrate as nonspecific perivalvular thickening (1). Such abscess and its subsequent complications including pseudoaneurysm and fistula imply locally uncontrolled infection, and are indications of urgent surgical intervention in case when medical treatment fails (3).
Recently, other imaging modalities including computed tomography (CT) (4), magnetic resonance imaging (MRI) (567), and 18F-fluoro-deoxyglucose positron emission tomography/CT (PET/CT) (89) have reported their potential roles in the early diagnosis of perivalvular abscess in patients with IE.
In this case report, we present a case of aortic perivalvular abscess detected by late gadolinium enhancement (LGE) in cardiovascular magnetic resonance (CMR) imaging, whereas initial transthoracic echocardiography only showed nonspecific perivalvular thickening.
A 66-year-old male man with known liver cirrhosis related to chronic hepatitis B presented with 10-day history of left scrotal pain, redness, and discharge. He was diagnosed of right communicating hydrocele and left indirect inguinal hernia by ultrasonography examination 5 months ago. Other than scrotal symptoms, he did not complained of any symptoms including fever nor dyspnea. He denied of any past history of neither cardiovascular surgery nor prosthetic device insertion. Physical examination was unremarkable except for the scrotum. His blood pressure was 106/64 mmHg, and electrocardiogram showed sinus rhythm, low voltage in frontal leads, and left axis deviation. Blood laboratory results revealed elevated white blood cell (WBC) count, 13,640 /µL; elevated serum C-reactive protein (CRP), 5.2 mg/dL; and elevated erythrocyte sedimentation rate (ESR), 38 mm/hr. Serum chemistry revealed hypoalbuminemia, 2.2 g/dL; and prothrombin time (PT) prolongation, 1.52 international normalized ratio (INR); probably due to underlying liver cirrhosis. Urine analysis revealed pyuria, and Klebsiella pneumoniae was detected in urine culture and in swab culture of the scrotal discharge. Blood culture was not done. Abdomen and pelvis CT demonstrated a large left scrotal abscess and right renal abscess. Intravenous (IV) antibiotic treatment (piperacillin-tazobactam, 4.5 g, every 8 hours) was started. Left orchiectomy with incision and drainage and ultrasonography-guided aspiration was done for the scrotal and the renal abscess, respectively. Klebsiella pneumoniae was detected in the culture of both left scrotal tissue and the aspirate from the renal abscess. IV antibiotic treatment was continued postoperatively.
TTE performed five days after initial presentation for preoperative evaluation before surgical repair of the left inguinal hernia showed thickened mitral-aortic intervalvular fibrosa, which was suspicious of infected mass or pseudoaneurysm (Fig. 1). Vegetation was not present. There was mild aortic valve regurgitation. TEE was not performed because of esophageal and gastric fundal varix secondary to the known liver cirrhosis.
CMR imaging was performed on a 1.5T scanner (Avanto, Siemens Medical Solution, Erlangen, Germany) with electrocardiographic gating, including pulse sequences as follows. Cine MR imaging with steady-state precession (TR = 47 ms, TE = 1.2 ms; slice thickness, 6 mm; acquisition matrix, 272 × 192; short-axis view, four-chamber view, two-chamber view), T1-weighted imaging (double inversion-recovery T1-weighted image with fat-saturation; TR = 925 ms, TE = 6.8 ms; slice thickness 6 mm), T2-weighted imaging (triple inversion recovery; TR = 1750 ms, TE = 81 ms; slice thickness 6 mm; acquisition matrix, 256 × 171), delayed contrast-enhanced imaging (phase-sensitive inversion recovery; TR = 700, TE = 1.17 ms; slice thickness 6 mm; acquisition matrix = 256 × 190, resolution 1.6 × 1.6 mm; time to inversion = 320 ms, 360 ms, 370 ms, respectively; image acquisition time after contrast administration = 8 min, 17 min, 19 min, respectively), IV gadobutrol 0.15 mmol/kg (Gadovist, Bayer Healthcare, Berlin, Germany).
CMR imaging showed normal left ventricle (LV) size and regional wall motion abnormality was absent. LV systolic function was within normal range, with ejection fraction (EF) measured 64.5%. Focal wall thickening between the anterior mitral leaflet and the non-coronary cusp of the aortic valve with late gadolinium enhancement on periphery (i.e., abscess wall) and central low signal intensity (i.e., abscess cavity) represented aortic perivalvular abscess (Fig. 2). Vegetation was not noted.
Despite lack of blood culture results, Klebsiella pneumoniae was assumed as the pathogen for the possible infectious endocarditis with perivalvular abscess. After 5 weeks of IV antibiotics treatment, follow-up TTE showed slight decrease in periaortic wall thickness with presence of a lucent focus. The patient was discharged uneventfully without surgical treatment.
Perivalvular abscess is the second most common finding in IE, and along with its complications such as pseudoaneurysm and fistula formation, is urgent indication of surgical intervention because of increased mortality rate (3). In echocardiography, abscess typically presents as hypoechoic area in perivalvular zone without detectable blood flow inside (2). TEE has higher sensitivity for detecting perivalvular abscess when compared to TTE, and therefore, TEE is recommended for the initial assessment of patients with IE and suspected perivalvular abscess. However, in early course of the abscess, only non-specific perivalvular thickening may be observed even in TEE, adding difficulty in prompt diagnosis (1). Furthermore, TEE may not be readily available depending on the patient factor such as risk of bleeding in esophageal varix or operator factor such as availability. TEE is an invasive procedure with rare but possible complications including oral benzocaine spray-related methemoglobinemia, Mallory-Weiss tear after intraoperative TEE, thoracic esophageal rupture, and fatal arrhythmia (7).
Recently, imaging modalities other than echocardiography has reported a potential roles in diagnosis of perivalvular abscess in patients with IE. CT finding of a markedly thickened area around the aortic root is indicative of an aortic root abscess, and may show good relation to TEE and pathologic findings (4). 18F-fluorodeoxyglucose PET-CT detects enhanced glucose metabolism within inflammatory or infectious lesions, and detection of perivalvular abscess has been demonstrated especially in the prosthetic valve grafts (8).
Few studies reported utilizing CMR imaging in diagnosis IE. Harris et al. (5) reported a case where CMR imaging was useful in defining perivalvular abscess associated with prosthetic aortic valve endocarditis. Sverdlov et al. (6) reported CMR confirming perivalvular abscess in its early stages with negative findings on both TTE and TEE. Dursun et al. (7) suggested that LGE of endothelial lining may contribute in the diagnosis of IE even in absence of vegetation.
In conclusion, perivalvular abscess is common and serious complications in patients with IE, and prompt diagnosis using echocardiography is warranted. Although further study must be done for validation of CMR imaging in diagnosis of IE, CMR imaging may provide complementary means in diagnosis of perivalvular abscess and evaluation of its extent when echocardiographic findings are elusive.
Figures and Tables
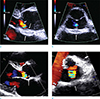 | Fig. 1Transthoracic echocardiography findings in a 66-year-old male. (a, b) Initial echocardiography shows thickening of aortic perivalvular area (arrows). (c, d) Follow-up study 37 days after the first echocardiography shows improved thickness of the lesion (arrows) and an echo-lucent focus (arrowhead). |
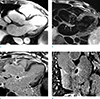 | Fig. 2MR imaging findings of perivalvular abscess in a 66-year-old male, two days after initial echocardiography. (a) 3-chamber view cine MR image shows focal wall thickening between the non-coronary cusp of the aortic valve and the anterior mitral leaflet (arrows). (b) 3-chamber view T2-weighted image shows slightly increased signal intensity of the focal wall thickening (arrows). (c, d) gadolinium contrast-enhanced delayed myocardial images demonstrate peripheral late gadolinium enhancements (arrows) with central hypointensity. |
References
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the american heart association. Circulation. 2015; 132:1435–1486.
2. Habib G, Badano L, Tribouilloy C, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010; 11:202–219.
3. Bruun NE, Habib G, Thuny F, Sogaard P. Cardiac imaging in infectious endocarditis. Eur Heart J. 2014; 35:624–632.
4. Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol. 2009; 53:436–444.
5. Harris KM, Ang E, Lesser JR, Sonnesyn SW. Cardiac magnetic resonance imaging for detection of an abscess associated with prosthetic valve endocarditis: a case report. Heart Surg Forum. 2007; 10:E186–E187.
6. Sverdlov AL, Taylor K, Elkington AG, Zeitz CJ, Beltrame JF. Images in cardiovascular medicine. Cardiac magnetic resonance imaging identifies the elusive perivalvular abscess. Circulation. 2008; 118:e1–e3.
7. Dursun M, Yilmaz S, Yilmaz E, et al. The utility of cardiac MRI in diagnosis of infective endocarditis: preliminary results. Diagn Interv Radiol. 2015; 21:28–33.
8. Saby L, Le Dolley Y, Laas O, et al. Early diagnosis of abscess in aortic bioprosthetic valve by 18F-fluorodeoxyglucose positron emission tomography-computed tomography. Circulation. 2012; 126:e217–e220.
9. Pizzi MN, Roque A, Fernandez-Hidalgo N, et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. 2015; 132:1113–1126.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download