INTRODUCTION
Wernicke's encephalopathy (WE) is a neurological disorder associated with thiamine deficiency (1). Patients may present with a classic triad of symptoms consisting of altered mental status, ataxia, and ocular dysfunction. Diagnosis can be difficult as clinical presentation is not typical in certain cases. WE is curable if thiamine is administered parenterally early in the course of the disease, but the mortality rate of WE remains 10-20% due to misdiagnosis (2). Although histopathologic examination usually demonstrates pin-point hemorrhages in affected brain parenchyma such as mammillary bodies, the periventricular regions of third and fourth ventricles and the aqueduct (3), secondary hemorrhage is a rare but serious complication of WE (4). We experienced a rare case of intracranial hemorrhage related to WE in a 56-year-old male patient with malnourishment.
CASE REPORT
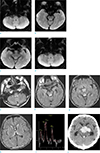
A 56-year-old man was admitted to our hospital with poor oral intake for a few months and sore formation in the right thigh due to bed rest state with quadriplegia. On admission, he had a mild fever (37.2℃), elevated WBC (27,520 cells/µl), decreased hemoglobin (10.2 g/dl), decreased platelet (18,000 µl-1), hematuria, and pyuria. Three weeks later, the patient suddenly showed decreased mental state. On diffusion weighted image (DWI) (Fig. 1a-d) and FLAIR images (Fig. 1e-h) of the brain, high signal lesions were seen in the bilateral mammillary bodies, tectal plate, periaqueductal gray matter of the midbrain, bilateral medial thalamic nuclei, and dorsal upper medulla. Magnetic resonance spectroscopy showed decreased NAA peak level in the medial thalamic nuclei which suggests that brain lesions were results from neuronal injury (Fig. 1i). These imaging findings were consistent with WE. On the basis of symptomatic and supportive treatments, the patient was given intravenous thiamine immediately. After 4 days since brain magnetic resonance imaging (MRI) work-up and thiamine administration, on 28 hospital days, at the neurological examination he was drowsy, limbs are flexia, and no response to pain stimuli. Then, we decided to perform a brain computed tomography (CT) scan. On brain CT, there were newly developed intracerebral hemorrhage (Intracranial hemorrhage) in the thalamus and periaqueductal gray matter and intraventricular hemorrhage (IVH) in the both lateral, third, and fourth ventricles (Fig. 1j). Emergency operation for removal of the ICH, IVH was performed and patient was taken care of in intensive care unit.
DISCUSSION
WE is characterized by acute onset of symptoms that may include confusion, dystaxia, and nystagmus. The MRI characteristically shows an increased T2/FLAIR signal that is bilaterally symmetrical in the paraventricular regions of the thalamus, the hypothalamus, mammillary bodies, the periaqueductal region, the floor of the fourth ventricle, and the midline cerebellum (5). However, the absence of imaging abnormalities does not exclude the diagnosis of WE that should be thought primarily from the clinical features and confirmed by symptomatic improvement with thiamine treatment. WE is a medical emergency, and if not recognized and treated promptly, it is associated with progression to irreversible Korsakoff psychosis, consisting of confabulation and anterograde memory deficits (6). The central nervous system is particularly sensitive to thiamine deficiency due to cellular dependence on oxidative metabolism. Even with early recognition and aggressive therapy, permanent disab-ility often occurs due to the irreversible cytotoxic effects on specific regions of the brain. Similar to this case, severe intracranial hemorrhage with WE give rise to the question that what the mechanism of the spontaneous bleeding in WE patient is and whether WE can raise risk of severe intracranial hemorrhage. Cases of intracranial hemorrhage in WE patient were seldom reported in the medical literature. One case, reported in 1995, showed intracranial hemorrhage with WE. In this case, small areas of hemorrhagic discoloration in cortical and subcortical regions at the walls of the third ventricle, the aqueduct and the floor of the fourth ventricle secondary to agonal raised intracranial pressure were proven by necropsy. And they showed prominent and dilated capillaries, reactive astrocytes in the periventricular regions, and mild neuronal loss (7). Another case, reported intracranial hemorrhage in WE patients in 1965, explained two factors of intracranial hemorrhage. The first factor is generalized bleeding diathesis not intrinsic to WE itself. The second factor is intrinsic to WE itself. This intrinsic factor affected the localization as well as the severity of the lesions seen in the disease, and hemorrhage, albeit only petechial or microscopic, may be part of these lesions. Thiamine deficiency is thought to play a key role in the genesis of the lesions, but the mechanism by which morphological alterations are brought about and the reasons for the selective vulnerability of the affected portions of the brain are not understood (8). One most intensive mechanical theory is suspected that when cell apoptosis and necrosis is occurred in WE, vascular structures are damaged together. And if combined with coagulopathy or anticoagulant therapy, severe intracranial hemorrhage can be occurred.
In conclusion, intracranial hemorrhage appears to be a rare complication in WE. We report a patient with massive intracranial hemorrhage as an unusual manifestation of WE and physicians should be aware of the possibility of massive intracranial hemorrhage in WE patients especially with underlying coagulopathy or anticoagulant therapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download