Abstract
Introduction
Distant metastases of mucoepidermoid carcinoma (MEC) are reported with the most common sites being the soft tissue of skin, lung, liver, and bone. We report here a very rare case of MEC with multiple metastases to the kidney, adrenal gland, skull and gluteus maximus muscle.
Case report
A 63-year-old male patient presented with left-sided headache. Radiologic evaluations including CT and MRI showed ill-defined soft tissue lesion involving the left infratemporal fossa and left sphenoid sinus, and multiple enlarged lymph nodes in neck and mediastinum. PET-CT demonstrated multiple hypermetabolic lesions in and around the left kidney, left adrenal gland, right ischium, right gluteus maximus and skull base. These lesions were confirmed as MEC with multiple metastases through biopsy.
Discussion
Only one case of metastasis to the skull has been previously reported, and moreover, there has not been a case of metastatic MEC to the kidney, adrenal gland and gluteus maximus muscle so far in the medical literature. It is important to acknowledge the possibility of every unusual MEC metastases, since the presence of metastasis has statistically significant influence on the survival of MEC.
Mucoepidermoid carcinoma (MEC) is one of the most common malignancies of the salivary glands (1). There are some reports of MEC with unusual distant metastases (234), but the reports of MEC metastases to the kidney, adrenal gland, skull and skeletal muscle have very rarely been observed. To the best of our knowledge, this is the first case report in which metastases to the kidney, adrenal gland and gluteus maximus muscle were observed. We report here a case of MEC which metastasized to these unusual sites.
A 63-year-old male patient presented with left-sided headache which had lasted for 2 months.
A computed tomography (CT) of the paranasal sinus showed ill-defined soft tissue lesion involving the left infratemporal fossa and left sphenoid sinus, associated with erosion and sclerosis of the left sphenoid bone. In addition, a CT of the neck demonstrated multiple enlarged lymph nodes with central necrosis in the right level IV, left level III-V and upper mediastinum (right paratracheal, prevascular, paraaortic and AP window lymph nodes) (Fig. 1).
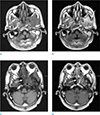
A magnetic resonance imaging (MRI) of the paranasal sinus also showed ill-defined soft tissue lesion involving the left infratemporal fossa and left sphenoid sinus with iso-signal intensity on T1-weighted image (T1WI). Contrast enhanced T1WI showed enhancement of the lesion (Fig. 2).
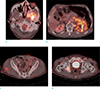
A positron emission tomography-computed tomography (PET-CT) showed increased standardized uptake values (SUV) in the multiple lymph nodes at various nodal stations (SUVmax, 7.8, left lower neck), in and around left kidney (SUVmax, 6.6), left adrenal gland (SUVmax, 5.1), right ischium (SUVmax, 4.4), right gluteus maximus (SUVmax, 2.7) and skull base (SUVmax, 5.6) (Fig. 3).
The serum levels of carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) level were elevated at 270.0 ng/mL and 72.0 U/mL, respectively.
Malignancy with multiple metastases was highly suggested with these overall findings, therefore the biopsy confirmation was recommended.
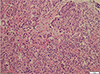
On endoscopic sinus biopsy, the left sphenoid lesion was composed of epidermoid cell nests with rarely scattered mucous cells, which are consistent with intermediate to high grade of MEC (Fig. 4). Excisional biopsy of the left lower neck lymph node and the left kidney showed metastatic carcinomas.
After receiving chemotherapy of 5-FU and cisplatin, follow-up CT scan revealed decreased extent of the soft tissue lesion involving left sphenoid sinus and decreased size and number of the metastatic lymph nodes, but the other metastatic lesions in the left kidney, left adrenal gland and right gluteus maximus muscle had increased in size.
MEC is one of the most common malignancies of the salivary gland, accounting for 3 to 15% of all salivary gland tumors (4).
Distant metastasis is rare in MEC (5). In approximately 15% of the patients with MEC, distant metastases are reported with the most common sites being the soft tissue of skin, lung, liver, and bone (6).
Recently, there were several reports showing MEC with other various kinds of distant metastases. Prabhu et al. (2) reported a 67-year-old man showing MEC of unknown primary with late distant metastasis to the triceps muscle, sacroiliac joint and thoracic spine. Sousa et al. (7) reported a very rare case of solitary skull metastasis from MEC. Onoda et al. (8) reported a 64-year-old man showing MEC of the pancreas, directly invading the spleen, left kidney, left adrenal gland and transverse colon, with distant metastasis to the left lateral lobe of the liver.
The presentation in this case was unusual because MEC metastases to the kidney, adrenal gland, skeletal muscle and skull are very rarely reported. MEC metastasis to the skull has not been reported, except in the case from Sousa et al. (7). To the best of our knowledge, this is the first case report in which distant metastases to the kidney, adrenal gland and gluteus maximus muscle were observed.
MEC is known as a cancer with a relative favorable outcome, the 5-year survival rate being more than 90%. However, the WHO defines it as 'carcinoma' since the number of reports on MEC with distant metastasis and fatal outcome is increasing (5). It is important to detect all the metastatic lesions because the presence of metastasis has statistically significant influence on the survival of MEC (9). Therefore, considering the possibility of unusual MEC metastases to the kidney, adrenal gland, skull or gluteus maximus muscle, as well as the common sites of MEC metastasis will be of help to predict the actual prognosis and to choose an appropriate treatment.
This report has a limitation that biopsy was not performed for the every suspicious metastatic lesions. Samples obtained from the left lower neck lymph node and the left kidney showed metastatic carcinomas, however, the exam was not done for the left adrenal gland and the right gluteus muscle. But considering that no other possible primary tumor origin was detected and that these lesions were highly suggestive of metastases on the PET-CT scan, they were most likely metastases from MEC.
In conclusion, we described an unusual case of MEC that spread to the multiple lymph nodes, left kidney, left adrenal gland, right gluteus maximus muscle and multiple skeletal bones including right ischium and skull base; these neoplasms were detected by CT, MRI and PET scanning, and they were confirmed by endoscopic sinus biopsy and excisional biopsy of the left lower neck lymph node and left kidney.
Figures and Tables
Fig. 1
A computed tomography (CT) of the paranasal sinus and neck. (a) Soft tissue window setting shows ill-defined soft tissue lesion involving the left infratemporal fossa. (b) Bone window setting shows abnormal soft tissue lesion in the left sphenoid sinus (arrows), associated with erosion and sclerosis of the left sphenoid bone (arrowheads). Multiple enlarged lymph nodes with central necrosis are shown in (c) the both level IV and (d) AP window. (Enlarged lymph nodes in other nodal stations are not shown here.) Fluid collection is also noted in left pleural space, but the patient has not undergone a pleural fluid analysis. It spontaneously resolved in a month.
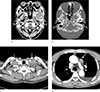
Fig. 2
A magnetic resonance imaging (MRI) of the paranasal sinus. Axial T1WI shows ill-defined soft tissue lesion involving (a) the left infratemporal fossa (arrow) and (c) left sphenoid sinus (arrow) with iso-signal intensity. (b, d) Contrast enhanced T1WI shows enhancement of the lesion (arrows).
References
1. Cho KJ, Kim JY, Lee SS, Oh KK. Mucoepidermoid carcinoma of the salivary gland--a clinico-pathologic and immunohistochemical study for c-erbB-2 oncoprotein. J Korean Med Sci. 1997; 12:499–504.
2. Prabhu V, Johnston J, Ingrams D, Passant C. Mucoepidermoid carcinoma - unknown primary and late distant metastasis: an unusual course of the disease. Clin Pract. 2011; 1:e97.
3. Suarez-Penaranda JM, Vieites B, Valeiras E, Varela-Duran J. Primary mucoepidermoid carcinoma of the skin expressing p63. Am J Dermatopathol. 2010; 32:61–64.
4. Gomes M, Pepe G, Bomanji J, et al. High-grade mucoepidermoid carcinoma of the accessory parotid gland with distant metastases identified by 18F-FDG PET-CT. Pediatr Blood Cancer. 2008; 50:395–397.
5. Friedrich RE, Klapdor R, Bartel-Friedrich S. Rapidly progressive and metastatic mucoepidermoid carcinoma: application of serological tumor markers. Anticancer Res. 2007; 27:2099–2100.
6. Healey WV, Perzin KH, Smith L. Mucoepidermoid carcinoma of salivary gland origin. Classification, clinical-pathologic correlation, and results of treatment. Cancer. 1970; 26:368–388.
7. Sousa J, Sharma RR, Pawar SJ, Mahapatra A, Mishra GP. Solitary skull metastasis from mucoepidermoid mimicking a parotid tumour. Neurol India. 2001; 49:194–196.
8. Onoda N, Kang SM, Sugano S, Yamashita Y, Chung YS, Sowa M. Mucoepidermoid carcinoma of the pancreas: report of a case. Surg Today. 1995; 25:843–847.
9. Roberto OL, Figueiredo SD, Oliveira-Costa JP, Sala DMA, Ribeiro-Silva A. Mucoepidermoid carcinoma of the salivary glands in Brazil: clinicopathological outcomes and a brief review. Revista Cubana de Estomatologia. 2012; 49:136–145.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download