Abstract
Benign fibrous histiocytoma (BFH) is a rare benign primary skeletal tumor that occurs commonly in the long bones, spine and pelvis. BFH constitutes a diagnostic challenge because it shares clinical background, radiological characteristics, and histological features with other fibrous lesions such as non-ossifying fibroma, giant cell tumor. We present a case of BFH with cystic change that occurred in the distal femur. We did not identify any case of BFH with cystic change involving the majority of the lesion that occurred in the metaepiphysis of the long bone.
Benign fibrous histiocytoma (BFH) is a rare primary skeletal tumor, with reported incidence of 1% of all surgically managed benign bone tumors (1). BFH is composed of fibroblasts and histiocytes that are arranged in a storiform pattern, which has almost identical histological features to non-ossifying fibroma (NOF) (2). Therefore, BFH is distinguishable from NOF by radiologic and clinical backgrounds (3). Almost all BFHs occur in patients older than 20 years, and the most are accompanied by pain (24). The radiologic findings are variable and not diagnostic; osteolysis, trabeculation, and bone sclerosis are seen, which may resemble other benign bone tumors. Involvement of an epiphysis or diaphysis in cases of BFH seems to have diagnostic importance, although metaphyseal localization (as in cases of NOF) has also been documented (5). In soft tissue BFH, central hemorrhage or cystic changes can be seen occasionally. In case of BFH of the bone, there is a report of cystic change of BFH developed in the calcaneus (6). Herein we report a rare case of BFH with hemorrhagic cystic change that occurred at the metaphysis-epiphysis of the distal femur, mimicking giant cell tumor (GCT).
A 31-year-old woman presented with one month history of pain affecting left knee. There was no previous trauma history to the knee. On physical examination, there was no evidence of swelling and tenderness on palpation. There was limitation of motion due to pain. The results of whole blood count and erythrocyte sedimentation rate were within normal range.
Plain radiograph demonstrated a 4.5-cm-size, eccentrically located, and radiolucent lesion at the metaphysis-epiphysis of the distal end of the femur. The lateral cortex was expansile, and it had a prominent marginal sclerosis in the medial aspect (Fig. 1). Computed tomography (CT) revealed a cystic lesion measuring 4.6 × 4.5 cm in diameter with a moderate expansion and thinning of the lateral cortex without soft tissue involvement.
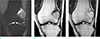
Magnetic resonance imaging revealed a heterogeneous signal intensity mass which was contoured bulging and located eccentric in the metaphysis of the distal femur extending to the epiphysis. The majority of the lesion consists of intermediate hyperintense material on T2- and proton density-weighted images, which was hyperintense on T1-weighted images without enhancement on the postcontrast image, suggestive of hemorrhagic components. In the superior portion of the tumor, there was curvilinear solid component which was hyperintense on T2-, hypointense on T1-weighted sequences and strongly enhanced on the postcontrast image. The radiologic diagnosis of GCT with hemorrhagic necrosis was suggested (Fig. 2).
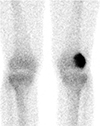
Bone scan showed intense uptake in left distal femur, suggesting osteoblastic activity (Fig. 3). A whole body fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET CT) scan showed mild hypermetabolic lesion at left distal femur (SUVmax = 4.4).
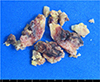
Intraoperative core needle biopsy was performed under C-arm fluoroscopic guidance, and pathology showed multifocal proliferation of mononuclear spindle cells with infiltrated foamy histiocytes and some scattered giant cells. The initial diagnosis of GCT was evoked on a histologically basis. Open bone curettage and cement packing was done 2 weeks after the initial curettage. The gross operative finding was a cystic lesion containing blood, which occupied over two-thirds of the volume of the lesion, but there was adjacent solid tumor. The surgical specimen was a gray-brown-colored, irregular lesion with areas of overt hemorrhage (Fig. 4). Histological examination revealed a tumor mainly composed of spindle-shaped fibroblastic cells arranged in a storiform pattern as well as histiocytes and admixed focally with scattered multinucleated giant cells. There were no mitotic figure, nuclear atypia, or necrosis. These features together with previously noted clinical and radiological findings were consistent with a histological diagnosis of BFH. The patient was discharged with no further complications. There was no evidence of disease after two years of follow-up.
BFH is a well-recognized tumor arising in the soft tissue, but it is a very rare bone tumor which accounts for about 1% of all surgically treated benign bone tumors (1). BFH occurs commonly in the spine, pelvis, facial bone or in tubular bones (578). However, there is a diagnostic challenge because the BFH histologically resembles NOF. Histologically, the BFH is composed of spindle-shaped fibroblasts arranged in a storiform pattern with varying degrees of multinucleated giant cells and foamy histiocytes (2). Therefore, it is suggested that BFH should not be distinguished from NOF on the basis of histologic characteristics but rather on clinical and radiologic grounds. Clinically in BFH, pain has to be considered as the principal symptom and a median age over 20 years is a considered factor that may differentiate BFH from NOF (3).
This case is a BFH in a 31-year-old female presented with symptom in affecting knee. NOF almost always develops in children and adolescents who are less than 20 years old and is considered to be developmental defects and self-limited processes (23). Clinically, NOF is typically asymptomatic except in case of pathological fracture and the lesion is found incidentally, whereas BFH frequently come to notice due to pain some months before (3). NOF spontaneously regresses with skeletal maturity, and surgical treatment is not needed mostly. Hemorrhagic or cystic change secondary to NOF appears to be extremely rare. Moreover, NOF is exclusively located in the metaphyses of the long bone. Therefore, the clinical findings, which is atypical for NOF could be the clues to the diagnosis of BFH.
Our case exhibited a radiologic appearance of bulging contoured, osteolytic lesion with partial sclerotic borders, eccentrically located in the distal femur. This presentation of mass, which was located in metaphysis of the long bone extending to epiphysis led us to think the possibility of GCT at the first time. From the radiologic standpoint, another differential possibilities include GCT, particularly when encountered metaphyseal-epiphyseal origin of tumor in adolescents and young adults (4). GCT is typically aggressive lytic lesion with poorly defined borders lacking surrounding rim of reactive sclerosis and sometimes with soft tissue extension, while BFH commonly reveals this feature. GCT always starts from the metaphysis, and the lesion seldom extends to the epiphysis before osseous maturity. GCT almost occurs when the growth plate has closed and are therefore typically seen in early adulthood, with 80% of cases reported between the ages of 20 and 50 (9). The most common presentation of GCT is pain, swelling, and limitation of joint movement at the primary site. In BFH, mass or swelling is not a frequent presenting symptom.
Microscopically, BFH consists of a variable amount of spindle-shaped fibrohistiocytic cells forming a storiform pattern, while GCT is composed of mononuclear cells intermixed with numerous uniformly distributed large giant cells containing up to 50 or more nuclei (9), much larger than those observed in other tumors. BFH may contain multinucleated giant cells and large areas of foam cells, lipid-filled cells with abundant vacuolated cytoplasm. However, GCT contains numerous thin walled vascular channels predisposing to areas of hemorrhage and presumably related to the relatively frequent co-existence of aneurysmal bone cysts found in 14% of cases. Moreover, malignant transformation and lung metastasis of GCT are possible, although rare. In contrast, BFH does not undergo malignant change or metastasis, although it can be locally aggressive and may recur after curettage (2).
On bone scintigraphy, BFH exhibits a moderately increased uptake, suggesting osteoblastic activity (7), also shown in our case. However, most GCT demonstrate increased uptake on delayed images, especially around the periphery, with a central photopenic region (doughnut sign). In GCT unlike BFH, increased blood pool activity is also seen, and can be seen in adjacent bones due to generalized regional hyperemia.
Magnetic resonance imaging demonstrates the lesion to be of intermediate signal intensity (isointense with the skeletal muscles) on T1-weighted sequences and of a high signal intensity on T2-weighted sequences (7). In our case, the majority region showing high signal intensity on T1- and T2-weighted sequences with no enhancement suggested hemorrhagic and cystic components, while upper small portion was hypointense on T1-, hyperintense on T2-weighted image with intense enhancement, suggestive of solid components. O'Donnell P, et al. (10) reported 33.3% of BFH of bone had fluid-fluid levels on MRI. However, the hemorrhagic area of the lesion was reported not large and the radiologic images were not available. A case of cystic BFH involving the calcaneus has been reported on CT, which mimicked cystic lesion such as GCT, simple bone cyst (6). However, we did not identify any case of BFH with cystic change occupying the majority of the lesion, which occurred in metaphysis-epiphysis of the long bone in the relevant literature.
Treatment consists of carefully complete curettage and filling of the defect with bone cement or graft materials (234). Prognosis is usually good, although recurrence has been reported (2). However, recurrence does not seem correlated with the surgical margin achieved, but rather with an incomplete removal of the tumoral tissue.
Figures and Tables
Fig. 1
Plain radiograph demonstrated an eccentrically located, and radiolucent lesion in the metaepiphysis of the distal femur. The lateral cortex was thinned and expansile. The osteolytic lesion had a partially marginal sclerosis along the medial border without intralesional matrix mineralization.
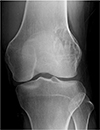
Fig. 2
Magnetic resonance imaging of BFH of the distal femur. (a) Coronal proton density-weighted image with fat suppression demonstrated an expansile heterogeneous mass in lateral aspect of the metaphysis of the distal femur extending to the epiphysis. The majority of the mass consists of intermediate hyperintense lesion with curvilinear, hyperintense component superiorly. (b) On T1-weighted coronal image, the majority of the mass appeared hyperintense, but the curvilinear part revealed hypointense superiorly. (c) On postcontrast image, the superior part of the mass showed strong enhancement meaning solid component, and the majority part of the mass showed no enhancement suggesting hemorrhagic component.
Acknowledgments
This paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2013.
References
1. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002; 114:56–63.
2. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985; 9:806–815.
3. Bertoni F, Calderoni P, Bacchini P, et al. Benign fibrous histiocytoma of bone. J Bone Joint Surg Am. 1986; 68:1225–1230.
4. Matsuno T. Benign fibrous histiocytoma involving the ends of long bone. Skeletal Radiol. 1990; 19:561–566.
5. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011; 95:107–114.
6. Keskinbora M, Kose O, Karslioglu Y, Demiralp B, Basbozkurt M. Another cystic lesion in the calcaneus: benign fibrous histiocytoma of bone. J Am Podiatr Med Assoc. 2013; 103:141–144.
7. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996; 25:25–29.
8. Pattamparambath M, Sathyabhama S, Khatri R, Varma S, Narayanan NM. Benign fibrous histiocytoma of mandible: a case report and updated review. J Clin Diagn Res. 2016; 10:ZD24–ZD26.
9. Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001; 21:1283–1309.
10. O'Donnell P, Saifuddin A. The prevalence and diagnostic significance of fluid-fluid levels in focal lesions of bone. Skeletal Radiol. 2004; 33:330–336.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download