Abstract
Acute Japanese encephalitis (JE) is an endemic viral infectious disease in various parts of Far East and Southeast Asian countries including Korea. Bilateral thalami are the most common involving sites in JE. Other areas including the basal ganglia, substantia nigra, red nucleus, pons, cerebral cortex and cerebellum may be also involved. We report an extremely unusual brain diffusion-weighted MR imaging (DWI) findings in a 53-year-old man with serologically proven JE involving unilateral deep gray matter and temporal lobe, which shows multifocal high signal intensities in left thalamus, left substantia nigra, left caudate nucleus and left medial temporal cortex on T2-weighted image and DWI with iso-intensity on apparent diffusion coefficient (ADC) map.
Japanese encephalitis (JE) is mosquito-borne flaviviral endemic encephalitis that remains to be an important health problem in some Far East and Southeast Asian countries including Korea and India. Most of the patients with JE present with nonspecific fever, flu-like symptoms, anorexia, nausea, vomiting, neck rigidity, hemiparesis, convulsions and/or altered mentality. About one third of the JE patients die and half of the survivors have severe neuropsychiatric sequelae. Children under 15 years of age are principally affected in endemic areas (1).
The brain MR imaging abnormalities of JE have been described as bilateral thalamic, substantia nigra, basal ganglia, brain stem, cerebellum, cerebral cortex and white matter lesions. The most consistent and characteristic MR imaging findings in JE is bilateral T2 hyperintensity in both thalami with or without hemorrhage and other lesions may be also noted in the areas described above (123). To our knowledge, unilateral involvement of a cerebral hemisphere in JE is extremely rare (4).
Here, we report an unusual case of serologically proven JE showing unilateral involvement in the thalamus, substantia nigra, caudate nucleus and medial temporal lobe on DWI.
A 53-year-old man with previously healthy state admitted via emergency room for fever and altered mentality. On arrival, his body temperature was 39.3℃, blood pressure was 139/84 mmHg and pulse rate was 112 beats/min. Initial his mental status was stuporous without neck stiffness and motor function decreased by all grade one.
Initial blood laboratory findings were normal with C-reactive protein (0.19 mg/dL), WBC (7500/uL) and segmented neutrophil (71.4%). Cerebrospinal fluid analysis (CSF) showed normal RBC count (0/uL) and raised WBC with dominant lymphocytes (15/uL) and protein (129 mg/dL) and normal glucose (59 mg/dL).
On non-enhanced brain computed tomography (CT) scan taken on hospital day 1, there was no intracranial abnormality. Imaging was performed on 1.5T MR scanners (Magnetom Avanto 1.5T, Siemens, Erlangen, Germany) taken on 2nd day of hospital admission and the patient had DWI performed with single-shot spin-echo echo-planar imaging (EPI) sequence, TR 4100 ms, TE 102 ms and b-value was set at 1000 s/mm2. Matrix size was 192 × 192 and the sequence was 5-mm-thick with a spacing of 2 mm. The DWI MR showed multiple areas of high intensity on DWI (b = 1000 s/mm2) and iso-intensity on apparent diffusion coefficient (ADC) map in left thalamus, substantia nigra, caudate nucleus and medial temporal lobe. T2-weighted images (DWI b = 0 s/mm2) showed subtle high intensity in the above regions (Fig. 1). Our differential diagnosis included JE, herpes simplex encephalitis (HSE) and Creutzfeldt-Jakob disease (CJD), or less likely, ischemic infarction.
The diagnosis of JE was confirmed by positive immunoglobulin M (IgM) antibodies to JE virus in the serum and CSF by IgM Antibody Capture Enzyme-Linked ImmunoSorbent Assay (MAC-ELISA, InBios) performed on day 14 after symptom onset. The result was reported as elevated index 57.75 and value of 1:256 of complement fixation IgM titer for JE virus establishing a diagnosis of JE.
The patient was clinically progressed to coma in mental state on hospital day 6. Brain CT scan performed on day 7 showed bilateral diffuse parenchymal low attenuation with poor gray-white matter differentiation in both cerebral hemispheres including the deep gray matter and some obliteration of all CSF spaces, but relatively preserved normal parenchymal attenuation in the cerebellum, suggesting either rapid fulminant progression of JE per se or diffuse hypoxic-ischemic encephalopathy. The patient showed fulminant disease course and died on hospital day 38.
JE is an endemic human encephalitis in various parts of Far East and Southeast Asian countries including Korea, Japan and India. JE virus is a single-stranded RNA flavivirus and the virus is transmitted to humans via infected culex mosquitoes.
JE is mostly a disease of children and young adults, even though the present case, a 53-year-old man, belongs to relatively elder age group. Patients with JE is often asymptomatic in the beginning and a few days later typically present with non-specific febrile illness, coryza, diarrhea, and rigors (5). When the patients progress to meningoencephalitic symptoms such as neck rigidity, cachexia, hemiparesis, and convulsions, they are usually associated with poor prognosis with a high mortality rate. If they survive, there is gradual recovery with or without persistent symptoms of neurologic injuries.
The diagnosis of JE is based on the essential and supportive criteria (1). According to the essential criteria, patient presents with acute encephalitic syndrome which is defined as a person of any age, at any time of year with acute onset of fever and a change in mental status and/or new onset of seizures. Supportive criteria include 1) patients coming from known JE endemic area, 2) detection of JE virus specific IgM in serum and CSF by the MAC ELISA, 3) thalamic lesions on CT or MRI scans in an appropriate clinical and epidemiological background. To confirm the disease, the patients fulfill the essential criteria and any two of the three supportive criteria (1).
Pathologic changes in the brains of acute JE patients are characterized by glial nodules and circumscribed necrolytic foci mainly in bilateral thalami, substantia nigra, basal ganglia, brain stem, cerebellum, cerebral cortical, and white matter (6) and the MR imaging findings reflect the pathologic changes in those areas (23). Among them, the most consistent characteristic findings in JE is bilateral involvement of both thalamic lesions with or without hemorrhage on MR imaging (3). But, unilateral lesion in JE has been extremely rarely reported (46). In a reported case (4), the patient with JE showed unilateral high intensity lesions in the left thalamus, substantia nigra and frontal lobe cortex on DWI.
Lesions in the temporal lobe in JE is also uncommon. Handique et al. (6) reported eleven (17.7%) of 62 patients in JE showed temporal lobe involvement with abnormal MR imaging. On the other hand, all patients had thalamic and substantia nigra involvement with basal ganglia involvement in 7 (11.3%). In the study (6), unilateral involvement was seen in the temporal lobe (n = 3), the thalamus (n = 1) and the substantia nigra (n = 1).
Temporal lobe involvement is much more common in HSE than in JE, which may cause problems in differentiating JE from HSE. Typical MR imaging findings in HSE is bilateral asymmetric T2 hyperintense in the limbic systems such as medial temporal lobes, insular cortices and inferolateral frontal lobes, regardless of hemorrhage and contrast enhancement. Unilateral temporal lobe involvement is not uncommon in HSE, but deep gray matter such as the thalamus, basal ganglia and substantia nigra are typically spared in HSE. Therefore, the concurrent involvement of the temporal lobe, thalami, substantia nigra, and basal ganglia is more likely to be JE rather than HSE (6).
Sawlani (7) reported the average ADC value is markedly low in acute HSE lesions which may reflect the abundance of cytotoxic edema which subsequently lead to cell death and necrosis, whereas the average ADC value wasn't significantly low in acute JE reflecting paucity of cytotoxic edema. Therefore, the average ADC value and diffusion abnormality may help in differentiating HSE and JE in case of overlapping imaging findings in endemic area (7). In our case, the ADC value was not significantly low and showed iso-intensity with high intensity on DWI (b = 1000 s/mm2).
In the present case, another imaging diagnostic problem was differential diagnosis from CJD. In patients with sporadic CJD (sCJD), the cerebral cortex and basal ganglia are typically involved in DWI and/or T2 FLAIR MR imaging (89). In a study of sCJD (10), the cerebral cortex was bilaterally involved in 91% (50/55), basal ganglia in 64% (35/55), thalamus in 11% (6/55) and cerebellum in 40% (22/55).
Patients with variant CJD (vCJD, mad cow disease) showed typical bilateral lesions in the posterior thalami and dorsomedial thalamic nuclei, which are called the pulvinar sign or hockey stick sign, respectively (810). There was no description on unilateral involvement in both sCJD and vCJD. In clinical practice, it is not difficult to differentiate CJD, regardless of sCJD or vCJD, from JE, because clinical symptoms of CJD are quite different from JE and there is no case of unilateral involvement reported in CJD (910).
In conclusion, we reported a rare case of JE with unilateral involvement of the thalamus, substantia nigra, caudate nucleus and medial temporal lobe on DWI. It is suggested that in the appropriate clinical setting of acute encephalitic syndrome, the concurrent unilateral involvement of the temporal lobe, thalami, substantia nigra and basal ganglia be more likely JE than HSE or other encephalitis, particularly in endemic area of JE.
Figures and Tables
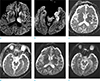 | Fig. 1A 53-year-old man with JE. Diffusion-weighted imaging (DWI) (b = 1000 s/mm2) (a, b), apparent diffusion coefficient (ADC) map (c, d) and T2-weighted image (DWI b = 0 s/mm2) (e, f) obtained on day 2 after onset of altered mentality show multiple areas of high intensity on DWI (b = 1000 s/mm2) (a, b) and isointensity on ADC map (c, d) in the left thalamus (short arrow), left caudate nucleus (long arrow), left subtantia nigra (arrowhead) and left medial temporal lobe (asterisk). On T2-weighted image (DWI b = 0 s/mm2, e, f), the lesions show subtle high intensity. |
Acknowledgments
We would like to express our sincere gratitude to Prof. Kee-Hyun Chang, MD for his assistance and advice in preparating and writing the manuscript.
References
1. Basumatary LJ, Raja D, Bhuyan D, Das M, Goswami M, Kayal AK. Clinical and radiological spectrum of Japanese encephalitis. J Neurol Sci. 2013; 325:15–21.
2. Kalita J, Misra UK. Comparison of CT scan and MRI findings in the diagnosis of Japanese encephalitis. J Neurol Sci. 2000; 174:3–8.
3. Kumar S, Misra UK, Kalita J, Salwani V, Gupta RK, Gujral R. MRI in Japanese encephalitis. Neuroradiology. 1997; 39:180–184.
4. Yakushiji Y, Kurohara K, Tanaka A, Kuroda Y, Uchino A. A case of Japanese encephalitis presenting with unilateral lesions in diffusion-weighted MRI. Rinsho Shinkeigaku. 2001; 41:602–605.
5. Solomon T. Viral encephalitis in Southeast Asia. Neurol Infect Epidemiol. 1997; 2:191–199.
6. Handique SK, Das RR, Barman K, et al. Temporal lobe involvement in Japanese encephalitis: problems in differential diagnosis. AJNR Am J Neuroradiol. 2006; 27:1027–1031.
7. Sawlani V. Diffusion-weighted imaging and apparent diffusion coefficient evaluation of herpes simplex encephalitis and Japanese encephalitis. J Neurol Sci. 2009; 287:221–226.
8. Hegde AN, Mohan S, Lath N, Lim CC. Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiographics. 2011; 31:5–30.
9. Meissner B, Kallenberg K, Sanchez-Juan P, et al. Isolated cortical signal increase on MR imaging as a frequent lesion pattern in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2008; 29:1519–1524.
10. Zeidler M, Sellar RJ, Collie DA, et al. The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Lancet. 2000; 355:1412–1418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download