Abstract
Carbon monoxide (CO) intoxication is a leading cause of the variable neuropsychiatric impairment. Despite of widely known central nerve system complications after CO intoxication, peripheral neuropathy due to CO poisoning is rare and has been under-recognized. We report interesting case of a 29-year-old male who suffered from motor weakness and sensory abnormalities in his lower extremity following acute CO intoxication. The patient revealed direct and indirect signs of peripheral neuropathy of the left inferior gluteal and sciatic nerve on magnetic resonance imaging.
Carbon monoxide (CO) is a colorless, odorless, and non-irritating gas produced as a result of incomplete combustion of carbon-containing fuels and materials. CO can affect all tissue organs including the brain, heart, kidney, skeletal muscle, skin, peripheral nerve, etc. (1). The clinical presentation of CO poisoning are variable and nonspecific. It is well-known about CO intoxication of brain related to impaired cognitive function, neuropsychiatric symptoms, and delayed neurological syndrome. But it has been rarely documented about the musculoskeletal complication after CO intoxication such as rhabdomyolysis, myonecrosis or peripheral neuropathy (12). To the best of our knowledge, image finding of peripheral neuropathy after CO intoxication has not even been described. So we reported a rare case of peripheral neuropathy in left lower extremity after CO intoxication with the imaging features of magnetic resonance imaging (MRI).
A 29-year-old male patient was transferred to an emergency department of outside hospital with stupor mental status. He was found beside the burned-out charcoal in bathtub. He was assumed to be exposed to the smoke from charcoal. Though serum carboxyhemoglobin was 36.3% (normal range; < 1.5% in non-smoker, 3-15% in smoker), the initial brain computed tomography (CT) and MRI revealed no significant abnormality in brain parenchyma including both globus pallidi. Because he did not have a history of stroke nor traumatic brain injury, he underwent hypothermia therapy and ventilator care with the assumptive diagnosis of CO intoxication. He recovered normal mentality the day after admission but complained of paresthesia, shooting pain and motor weakness of his left lower extremity. The pain existed from thigh to foot, but dominant along the thigh at that time. Straight leg raising test and femoral stretch test were negative. He had conservative treatment with medication and finally discharged two weeks later with slightly improved left leg symptom.
2 weeks after discharge, the patient re-visited the emergency department due to the disorientation of location, date and calculation. He scored 23/30 on Korean-mini mental state examination (K-MMSE). He also suffered from continuous left leg pain with motor weakness and transferred to our hospital for further evaluation of left leg problem and hyperbaric oxygen (HBO) therapy.
On physical examination, the patient revealed decreased motor power during extension of left hip, flexion and extension of left knee and ankle (grade 3). He showed pathologic reflex of knee and ankle jerk, ankle clonus and calf tightness. In terms of paresthesia along the whole left lower extremity, the sole side of foot was most annoying site he complained.
With brief mention of follow-up brain MRI performed 8 weeks after initial CO intoxication, there are ill-defined hyperintensity lesions in both periventricular white matter, corpus callosum and centrum semiovale on T2-weighted image (T2WI) and fluid-attenuated inversion recovery (FLAIR) with focal diffusion restriction along the splenium of corpus callosum (Fig. 1). So clinically and radiologically presumed diagnosis of the patient was delayed hypoxic leukoencephalopathy and associated neuropsychiatric symptoms.
Simultaneously with HBO therapy, a lumbosacral spine MRI was done for evaluating his leg pain. Spine MRI only revealed mild central disc protrusion in L4-5, which could not sufficiently explain for his severe left leg pain.
Electrophysiologic study (EPS) was performed in the department of rehabilitation. On needle electromyography (EMG), the abnormal spontaneous fibrillations and positive sharp waves were noted along the left tibialis anterior and peroneus longus muscles which are innervated by deep and superficial peroneal nerve. The medial head of left gastrocnemius and abductor hallucis muscle innervated by tibial nerve and short head of biceps femoris muscle innervated by sciatic nerve also revealed abnormal spontaneous fibrillations and positive sharp waves. These involved muscles are generally innervated by peroneal compartment of sciatic nerve. On motor nerve conduction studies (NCS), the conduction velocity of left common peroneal nerve was markedly decreased with low amplitude (0.4-1.5 mV).
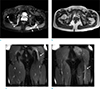
On subsequently performed non-enhanced lower extremity MRI, left sciatic nerve was swollen and revealed high signal intensity on fat-suppressed T2WI from the level of greater sciatic foramen to distal proximal thigh in scanned level. And there was abnormal high signal intensity on fat-suppressed T2WI in the superomedial aspect of left gluteus muscle, representing denervation edema by the branch of inferior gluteal nerve, though any abnormal finding of inferior gluteal nerve was not visible on MRI (Fig. 2). On the right side, right obturator internus muscle revealed intramuscular high signal intensity on fat-suppressed T2WI (Fig. 2). There was no evidence of extrinsic compression or space occupying lesion along the course of both lumbosacral plexus.
Though the EPS of right leg and MRI of left lower leg were absent, the overall result of these studies suggested peripheral neuropathy involving the branches of left inferior gluteal nerve and left sciatic nerve and possibility of right side peripheral neuropathy on nerve branch to obturator internus from L5-S2.
Overall, our patient had a low chance to have a compressive mononeuropathy because there was no evidence of any extrinsic compression to sciatic nerve nor the involved hip muscles. Also it is not proper to assume metabolic toxicity which can damage to hip muscles or sciatic nerve, because the patient was young-healthy male who did not have any underlying disease and there was no evidence of electrolyte imbalance except elevated serum carboxyhemoglobin on initial laboratory test. At last, post-injection sciatic nerve injury may be considered as another cause of sciatic neuropathy. However, our patient had no intramuscular gluteal injections before the symptom onset. For diagnosis of exclusion, it is reasonable to presume that CO intoxication is responsible for the peripheral neuropathy in this case.
The patient had undergone rehabilitative exercises and medication for pain control during 6 days and discharged against medical advice. With intermittent conservative treatment including nonsteroidal anti-inflammatory drug and hot pack massage, the extent and degree of left lower leg pain and numbness has markedly decreased during about 2 years since the first symptom onset (VAS 8 → 1). And he got full recovery in motor power of left lower extremity. Now the remnant annoying symptom is localized on the sole side of left foot with intermittent mild paresthesia.
CO competes with oxygen and binds strongly with hemoglobin to form carboxyhemoglobin, which results in shifting the oxyhemoglobin dissociation curve to the left. The affinity between CO and hemoglobin is 200 times as high as that of oxygen. CO can affect nearly all tissue organs and numerable bibliographic references were reported particularly within the frame work of central nerve system. Though many cases of acute CO intoxication can reveal normal brain MRI on acute phase, the bilateral globus pallidi lesions are widely known to be a typical brain imaging finding of acute phase. On the other hand, cerebral white matter lesions tend to be shown in a few days or a few weeks after CO intoxication. It is so called delayed hypoxic leukoencephalopathy (DHL) and the CO intoxication is known to be the most common cause of DHL. But peripheral neuropathy has relatively been under-recognized.
In terms of CO-mediated peripheral neuropathy, CO-induced hypoxia can aggravate impairment of oxidative metabolism in affected tissue such as central or peripheral nerve systems (3). It may either result in reversible demyelination by lipid peroxygenation or permanent failure to meet the energy requirements necessary to sustain cellular integrity by such as mitochondrial oxidative stress (34). CO itself may also be toxic to peripheral nerves and binds to platelet heme protein and cytochrome c oxidase, interrupting cellular respiration and causing oxidative stress, which in turn leads to neuronal necrosis, and apoptosis, contributing to inflammation and injury (3).
In terms of the clinical manifestation and the prognosis of peripheral neuropathy, it depends on which type of peripheral nerves are damaged (sensory, motor or autonomic nerves). Neuropathy can affect any one type, or a combination of all three types, of nerves. It is same as in sciatic neuropathy. Sciatic nerve derives from lumbosacral plexus (L4 to S3) and divided into tibial and common peroneal nerve. It supplies sensation to the skin of the foot, as well as the entire lower leg except for its inner side. The sciatic nerve also innervates muscles particularly the muscles in the posterior compartment of the leg and plantar aspect of the foot via tibial nerve and the muscles in the anterior and lateral compartments of the leg via the common peroneal nerve. So when the sciatic nerve damaged, variable clinical manifestation may be possible including sensory or motor or combined neuropathy which depends on the degree, distribution or function of affected nerve fiber.
According to a study on 20 cases of peripheral neuropathy after CO intoxication by Choi et al. (1), the incidence of peripheral neuropathy was less than 1% and usually occurred in young adults (mean age; 29.5 years). The lower extremities, especially the left side, were vulnerable to peripheral neuropathy. Symptoms were usually sensory only, but motor or even combined forms were noted (12). Our patient was also healthy young male who represented left lower extremity peripheral neuropathy particularly involving sciatic nerve. But he revealed both motor and sensory symptoms.
Considering in regard to the prognosis of peripheral neuropathy following CO intoxication, Choi's study reported fifteen cases among 20 cases had full recovery within 3 to 6 months. Another case report of bilateral brachial plexus injury following acute CO intoxication by Rahmani et al. (5) 42 years old male who showed a brachial diplegia and hypoesthesia had complete recovery of neurological disorders. On the other hand, Jeong et al. (6) reported a case of bilateral femoral neuropathy after CO intoxication which showed persisted neuropathy with minor improvement. In our case, the patient had full recovery of motor power and marked improvement of sensory symptoms except mild residual intermittent paresthesia localized on the sole.
As we know, ultrasound or CT has large limitation in the evaluation of soft tissue lesions, so the high resolution MRI is useful not only in diagnosis but also detection of extent of affected structures in peripheral neuropathy. Typical image findings of peripheral neuropathy are divided into direct and indirect signs. Direct signs of peripheral neuropathy are the changes of affected nerve itself, such as changes in signal intensity particularly high signal intensity on T2WI, variable but contrast enhancement in usual, increased diameter of nerve by swelling, morphologic distortion, etc. Indirect sign of involved muscle is characterized by increased muscle signal intensity on fluid-sensitive MR sequences as a result of acute or subacute denervation edema, which also may be an important contributing factor to the development of neuropathy (5). Sequentially the affected muscle may reveals increased signal intensity on T1WI with decreased volume due to fatty atrophic change (78). Peripheral neuropathy by acute CO intoxication in our case showed both direct and indirect changes on MRI.
Traditionally, the diagnosis of peripheral neuropathy is dependent on the bases of physical and neurologic examination, electromyography and nerve conduction studies. But these examinations retain some weak point such as patient's pain during the test, low reproducibility in result or interobserver bias. So the imaging studies are increasingly important for primary and supplementary modality of accurate diagnosis in peripheral neuropathy. There is a study, already reported, about the correlation between the quantitative EMG and signal change on MRI (7). By the study of Jonas et al. (7), a high correlation was found between the amount of pathologic spontaneous activity (PSA) on EMG and the T2-weighted signal intensity in short tau inversion recovery (STIR) sequence. So the MRI demonstrates the structural changes and thus visualizes the outcome of the functional changes of denervation detected by EMG. In our case, we ascertain the relationship between the clinical features of peripheral neuropathy with abnormal EMG findings and abnormal high signal intensity of affected nerve and muscle on fat-suppressed T2WI, though we could not obtain the quantitative analysis of positive correlation between the amount of PSA and T2 hyper signal intensity. Furthermore, we can also evaluate the serial changes on imaging features by reinnervation detected on EMG (9). But unfortunately, our patient have not underwent follow-up MRI.
In summary, reversible peripheral neuropathy should be considered as a one of the possible complication after acute CO intoxication. And, as this case, the MR findings suggesting non-compressive peripheral neuropathy with neuritis and denervation change of muscles can be seen.
Figures and Tables
Fig. 1
On fluid-attenuated inversion recovery (FLAIR) images of follow-up brain MRI 4 weeks after CO intoxication (a, b), ill-defined high signal intensity lesions are seen along the both periventricular white matters, centrum semiovale (white arrows) and splenium of corpus callosum (arrowhead). On Diffusion-weighted image, focal high signal intensity is seen in the splenium of corpus callosum with corresponding low ADC map (arrowheads) (c, d), representing a b diffusion restriction.
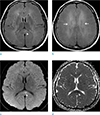
Fig. 2
On lower extremity MRI, axial fat-suppressed T2-weighted image (a) and T1-weighted image (b) show swollen left sciatic nerve with T2 hyperintensity (arrow). On fat-suppressed T2-weighted image (a), ill-defined high signal intensity lesions (arrowheads) are seen in left gluteus maximus and right obturator internus muscles, representing denervation edema. Two coronal fat-suppressed T2-weighted images (c, d) also reveal signal changes with swelling of left sciatic nerve at the levels of buttock and thigh (arrows).
References
1. Choi IS. Carbon monoxide poisoning: systemic manifestations and complications. J Korean Med Sci. 2001; 16:253–261.
2. Choi IS. A clinical study of peripheral neuropathy in carbon monoxide intoxication. Yonsei Med J. 1982; 23:174–177.
3. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009; 360:1217–1225.
4. Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest. 1992; 90:1193–1199.
5. Rahmani M, Belaidi H, Benabdeljlil M, et al. Bilateral brachial plexus injury following acute carbon monoxide poisoning. BMC Pharmacol Toxicol. 2013; 14:61.
6. Jeong HJ, Kim CH, Kim MO, Jung HY. Bilateral femoral neuropathy in carbon monoxide poisoning: a case report. J Korean Assoc EMG Electrodiagn Med. 2011; 13:73–76.
7. Jonas D, Conrad B, Von Einsiedel HG, Bischoff C. Correlation between quantitative EMG and muscle MRI in patients with axonal neuropathy. Muscle Nerve. 2000; 23:1265–1269.
8. Andreisek G, Crook DW, Burg D, Marincek B, Weishaupt D. Peripheral neuropathies of the median, radial, and ulnar nerves: MR imaging features. Radiographics. 2006; 26:1267–1287.
9. Wessig C, Koltzenburg M, Reiners K, Solymosi L, Bendszus M. Muscle magnetic resonance imaging of denervation and reinnervation: correlation with electrophysiology and histology. Exp Neurol. 2004; 185:254–261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download