Abstract
Clear cell sarcoma is rare and difficult to diagnose. Herein, we present a case of clear cell sarcoma in the dorsum of the wrist with MRI findings, including diffusion-weighted imaging, and histopathologic correlation, which was initially diagnosed as giant cell tumor of tendon sheath.
Clear cell sarcoma (CCS, malignant melanoma of soft parts) is a rare neoplasm accounting for 1% of all soft tissue sarcomas (1). Young adults between the ages of 20 and 40 are most commonly involved (1). CCS is intimately associated with or in a tendon, ligament, or aponeurosis. Suggestive features of diagnosis best seen on MR imaging include a mass having components not only surrounding but within a tendon, ligament, or aponeurosis. Herein, we describe a case of CCS of the dorsum of the wrist closely abutting the tendons with MRI-histopathologic correlation including diffusion-weighted imaging.
A 54-year-old woman was admitted with a painless mass on the dorsum of her left wrist. The mass was first found 4 years ago and noticeably grew recently. The lesion became larger while the patient was lifting heavy things or doing hard work, and got smaller while she was resting, so the clinician gave an impression of hemangioma.
Preoperative MRI showed a well-marginated, lobulated mass at the dorsum of the left wrist, which was abutting to and partly encasing the common extensor digitorum tendons, with extension to tendon sheaths of extensor carpi radialis brevis (ECRB) and extensor carpi radialis longus (ECRL). The lesion measured 4.0 × 1.3 × 3.3 cm in size and showed intermediate to high signal intensity on T1-weighted images (T1WI), slightly heterogeneous intermediate to high signal intensity on T2-weighted images (T2WI), and heterogeneous enhancement. The lesion showed high signal intensity for the most part on diffusion-weighted image and mean apparent diffusion coefficient (ADC) value of 0.93 × 10-3 mm2/sec of on ADC map (b = 0, 40, and 1400 sec/mm2) (Fig. 1).
Based on the history, location, and MR imaging findings, the lesion was initially diagnosed as a benign tumor, such as giant cell tumor of tendon sheath. Upon surgery, the surgeon found that the connective tissue of the mass and extensor digitorum tendon sheath were connected, so the surgeon performed a wide excision, including parts of the tendon sheath of the extensor digitorum tendons. Our pathologist suggested the diagnosis to be CCS (malignant melanoma of soft parts) on basis of histological features and immunohistochemistry results.
Grossly the tumor was a localized, firm, and circumscribed mass, measuring about 4.2 × 3.3 × 2.3 cm in size. Clusters or nests consist of tumor cells with surrounding abundant fibrovascular stroma were demonstrated on Hematoxylin & Eosin stain (Fig. 2a); the tumor cells had abundant clear cytoplasm and central nucleus with prominent nucleoli. On immunohistochemical studies, these neoplastic cells showed immunoreactivity for HMB-45, melan-A, vimentin and S-100 protein (Fig. 2b).
Real-time polymerase chain reaction for BRAF and c-KIT mutations showed negative results. Although it is recommended to confirm translocation of EWS gene and EWS-ATF1 fusion gene to differentiate CCS with malignant melanoma, we performed PCR for BRAF and c-KIT mutations only.
After pathologic confirmation, the patient got follow-up studies and further evaluation including PET-CT. Axillary lymph node metastasis was found and she underwent adjuvant radiation therapy.
Enzinger first proposed the term CCS of tendons and aponeuroses in 1965, as a rare malignant tumor originating from tendons and aponeurosis, with histological clear cell appearance due to the accumulation of glycogen (1). In 1973, melanocytic differentiation was recognized by the presence of cytoplasmic melanosomes (2). In 1983, the name "malignant melanoma of soft parts" was proposed due to its histological similarities to malignant melanoma. Thereafter, a number of papers have been published describing this disease entity. As a result, CCS (malignant melanoma of soft parts) has been described as a rare neoplasm accounting for 1% of all soft tissue sarcomas.
Young adults between the ages of 20 and 40 are most commonly involved. In the extremities, CCS can be intimately associated with a tendon, ligament, or aponeurosis (1). The lower extremity is involved in 60% to 75% of cases with a particular predilection for the foot/ankle (43% of cases) followed by the knee and thigh (1). Involvement of the hand (18% of cases) and wrist is most frequent in upper extremity lesions, paralleling the distribution in the lower extremity. Suggestive features of diagnosis best seen on MR imaging include a mass having components not only surrounding but presenting within a tendon, ligament, or aponeurosis.
On initial diagnosis, we should have considered CCS as one of the differential diagnosis, but due to its low prevalence and clinical findings, such as age and insidious nature, we suggested giant cell tumor of tendon sheath as our first impression. Our case in retrospect showed relatively higher signal intensity on T1-weighted images. According to De Beuckeleer et al. (3), the presence of this relative higher signal intensity on T1-weighted images is rather specific for tumors displaying melanocytic differentiation. Low signal intensity on long TR MR images and high signal intensity on T1-weighting in CCS has been attributed to the effects of melanin (paramagnetic relaxation enhancement of surrounding tissues) (1). Theoretic effects of melanin is not prominent on MR imaging, because of the minute amount of melanin, immunochemical stains are required for detection of it. For these reasons, radiologists should familiarize themselves with this rare entity and include it in their differential diagnosis when confronted with a well-defined, homogeneous, strongly enhancing mass with slightly higher signal intensity compared with muscle on pre-contrast T1-weighted images.
Maeda et al. (4) found no significant difference between the ADC values of malignant and benign soft tissue tumors. Razek et al. (5) reported that malignant tumors tend to exhibit a lower mean ADC value than benign soft tissue tumors and proposed using a threshold mean ADC value of 1.34 × 10-3 mm2/sec to help distinguish benignity from malignancy. As far as we know from the current literature, there has been no report about findings of CCS on diffusion-weighted image. In our case, tumor showed diffusion restriction on diffusion-weighted image for the most part and an average ADC value of 0.93 × 10-3 mm2/sec on ADC map. Compared with the results in previous studies, mean ADC value of the lesion of our case is lower than proposed threshold (mean ADC value of 1.34 × 10-3 mm2/sec), which favors a malignant soft tissue tumor (5).
Histopathologically, CCS displays a number of features exhibiting a broad morphologic overlap with conventional malignant melanoma, including melanin synthesis and expression of HMB-45 and S-100 protein (6) and CCS is cytogenetically characterized by the t(12;22)(q13;q12) resulting in the chimeric EWSR1/ATF1 gene (7). Therefore, it could be considered as a limitation of this case that our institution has not confirmed translocation t(12;22) (q13;q12) EWS-ATF-1.
However, BRAF and KIT mutations are well-known melanocytic tumorigenesis-associated mutations frequently found in cutaneous melanoma, but are normally rare or absent in CCS (8). Although there are a few cases have been reported about CCSs with BRAF or KIT mutations, those cases are very rare and, therefore, our pathologist made a diagnosis CCS on the basis of the histochemistry and negative results of BRAF and KIT mutation studies.
In conclusion, when we evaluate soft tissue tumors of the wrist and hand, we should consider CCS (malignant melanoma of soft parts) as a possible diagnosis, especially in a case, when the tumor demonstrates intermediate to high signal intensity on T1- and T2WI compared with signal intensity of muscle and relatively low mean ADC value (lower than 1.34 × 10-3 mm2/sec), even if the lesion is closely abutting to the tendon.
Figures and Tables
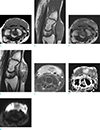 | Fig. 1A 54-year-old woman with clear cell sarcoma (malignant melanoma of soft parts) on the dorsum of the left wrist. (a) Axial and (b) sagittal T1-weighted images, (c) axial and (d) sagittal T2-weighted images demonstrate a well-marginated, lobulated mass with heterogeneous intermediate to high signal intensity compared with muscle. (e) Post-contrast image (TSE, TR/TE 720/12) clearly shows the heterogeneously enhancing mass at the dorsum of the wrist. (f) Corresponding axial ADC map (b = 0, 400, and 1400 sec/mm2) shows an area of low signal intensity that correspond to the tumor, with minimum and average ADC values of 0.52 × 10-3 mm2/sec and 0.93 × 10-3 mm2/sec, respectively. (g) Diffusion-weighted axial image (b = 1400 sec/mm2) shows high signal intensity for most part of the lesion. |
References
1. Kransdorf MJ, Murphey M. Imaging of soft tissue tumors. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;a WOLTERS KLUWER business;2014. p. 435–438.
2. Hoffman GJ, Carter D. Clear cell sarcoma of tendons and aponeuroses with melanin. Arch Pathol. 1973; 95:22–25.
3. De Beuckeleer LH, De Schepper AM, Vandevenne JE, et al. MR imaging of clear cell sarcoma (malignant melanoma of the soft parts): a multicenter correlative MRI-pathology study of 21 cases and literature review. Skeletal Radiol. 2000; 29:187–195.
4. Maeda M, Matsumine A, Kato H, et al. Soft-tissue tumors evaluated by line-scan diffusion-weighted imaging: influence of myxoid matrix on the apparent diffusion coefficient. J Magn Reson Imaging. 2007; 25:1199–1204.
5. Razek A, Nada N, Ghaniem M, Elkhamary S. Assessment of soft tissue tumours of the extremities with diffusion echoplanar MR imaging. Radiol Med. 2012; 117:96–101.
6. Kindblom LG, Lodding P, Angervall L. Clear-cell sarcoma of tendons and aponeuroses. An immunohistochemical and electron microscopic analysis indicating neural crest origin. Virchows Arch A Pathol Anat Histopathol. 1983; 401:109–128.
7. Panagopoulos I, Mertens F, Isaksson M, Mandahl N. Absence of mutations of the BRAF gene in malignant melanoma of soft parts (clear cell sarcoma of tendons and aponeuroses). Cancer Genet Cytogenet. 2005; 156:74–76.
8. Park BM, Jin SA, Choi YD, et al. Two cases of clear cell sarcoma with different clinical and genetic features: cutaneous type with BRAF mutation and subcutaneous type with KIT mutation. Br J Dermatol. 2013; 169:1346–1352.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download